Providence, RI – Providence-based biotech company EpiVax, Inc. was awarded a new $55,000 grant from the GBS-CIDP Foundation International, to explore using Tregitopes as a novel immuno-modulator therapy for a nerve disease that is currently treated with intravenous immunoglobulin G (IVIG). This award and the recent addition of an SBIR grant for $600,000 to explore the use of Tregitope for Pompe disease (August 2012) will bring the total amount of funding awarded to EpiVax for research and development of Tregitopes to more than $3.4M this year. The surge in funding will result in new hires at the Providence-based biotech company, which is considering spinning off the Tregitope technology should angel or venture funding be made available. Interested applicants should visit the EpiVax.com website and send in their CV.
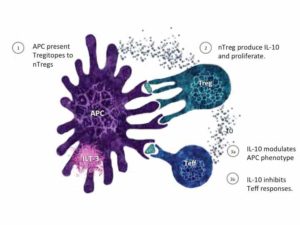 This additional funding for an Orphan Disease indication will compare Tregitope with IVIG. The new research funds will be applied to developing a novel therapy for the treatment of individuals who have CIDP, also known as Chronic Inflammatory Demyelinating Polyneuropathy. Patients develop a debilitating and painful nerve condition that requires treatment with IVIG, among other medications. A single dose of IVIG costs $8,000, and patients often spend tens of thousands of dollars obtaining treatment for their condition per year. Alternative treatments that might redirect the immune response toward antigen-specific tolerance without immunosuppressive agents are needed. With funding from the NIH SBIR program, Tregitopes (T regulatory epitopes) will be tested, in animal models, for the future treatment of CIDP. Tregitopes effect with IVIG, the current therapy for CIDP. Other diseases that can be treated with IVIG include Guillain-Barre syndrome, and IVIG therapy is now under study for the treatment of Alzheimer’s. Scientists at EpiVax believe that the mechanism of action of IVIG and Tregitopes are very similar, if not identical in some cases.
This additional funding for an Orphan Disease indication will compare Tregitope with IVIG. The new research funds will be applied to developing a novel therapy for the treatment of individuals who have CIDP, also known as Chronic Inflammatory Demyelinating Polyneuropathy. Patients develop a debilitating and painful nerve condition that requires treatment with IVIG, among other medications. A single dose of IVIG costs $8,000, and patients often spend tens of thousands of dollars obtaining treatment for their condition per year. Alternative treatments that might redirect the immune response toward antigen-specific tolerance without immunosuppressive agents are needed. With funding from the NIH SBIR program, Tregitopes (T regulatory epitopes) will be tested, in animal models, for the future treatment of CIDP. Tregitopes effect with IVIG, the current therapy for CIDP. Other diseases that can be treated with IVIG include Guillain-Barre syndrome, and IVIG therapy is now under study for the treatment of Alzheimer’s. Scientists at EpiVax believe that the mechanism of action of IVIG and Tregitopes are very similar, if not identical in some cases.
Based on funding received by EpiVax this year, it is clear that Tregitopes may have broad applications in autoimmune diseases such as Multiple Sclerosis (MS). EpiVax is also working with Samia Khoury M.D., an internationally recognized MS researcher based at Harvard Brigham and Women’s hospital, to develop a Tregitope-based treatment for MS. Dr. Khoury has said that that pre-clinical studies of Tregitope being carried out in her laboratory by researcher Wassim Elyaman, Ph.D., are “promising”.
Read more about Tregitope here.
Read more about CIDP and Guillain Barré, and the organization funding this research here.
PDF of this news release is available here.

Trackbacks/Pingbacks