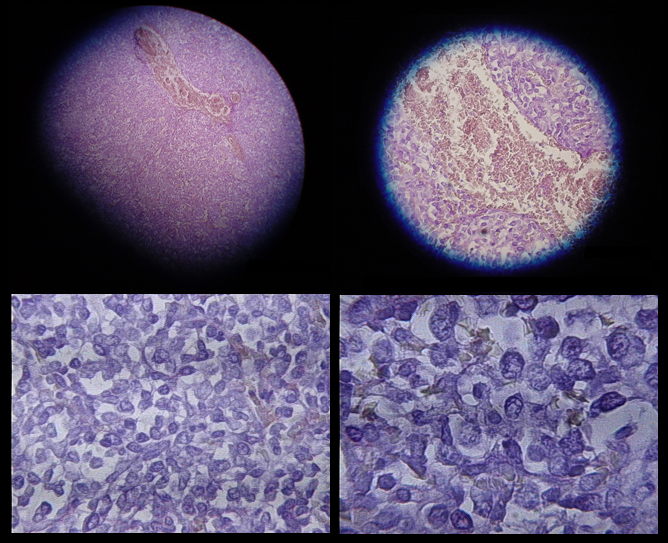
Time for T? Immunoinformatics addresses the challenges of vaccine design for neglected tropical and emerging infectious diseases
Expert Review of Vaccines. Resubmitted 3 Aug 2014. Full Text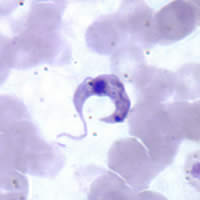
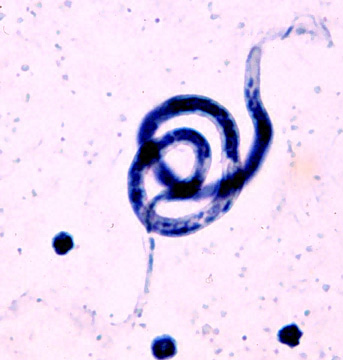
An immunoinformatic approach for identification of Trypanosoma cruzi HLA-A2-restricted CD8+ T cell epitopes
Hum Vaccin Immunother. 2015;11(9):2322-8 .PMID: 26107442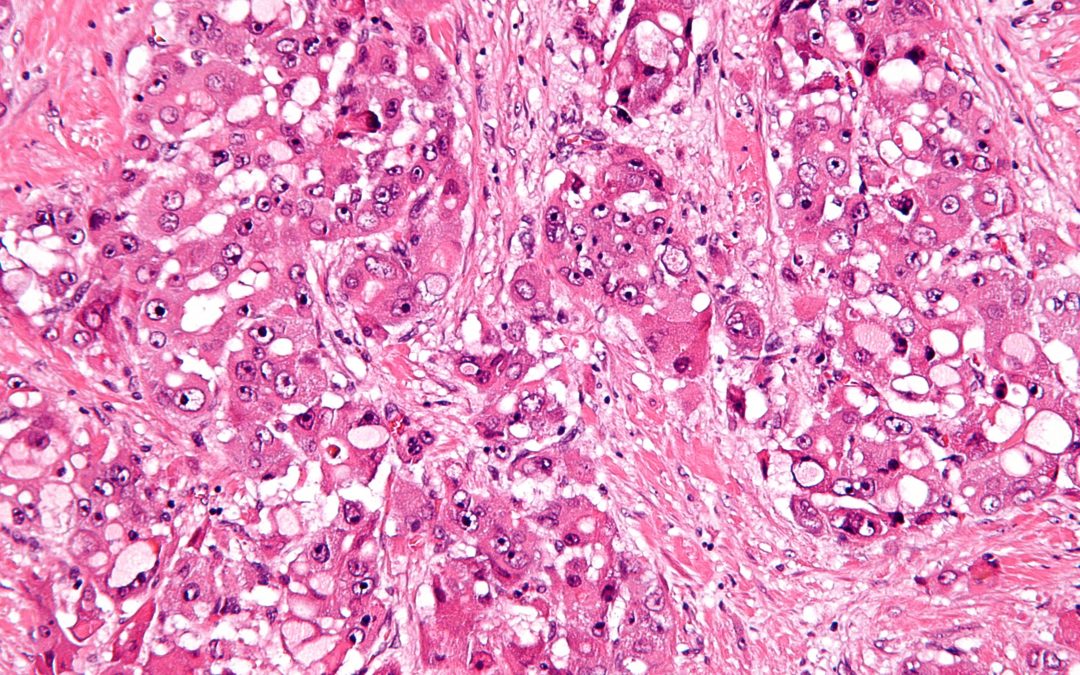
Aspartate-β-hydroxylase induces epitope-specific T cell responses in hepatocellular carcinoma
Vaccine. 2015;33(10):1256-66 PMCID: PMC4331251