
A SARS-CoV-2 NSP7 homolog of a Treg epitope suppresses CD4+ and CD8+ T cell memory responses

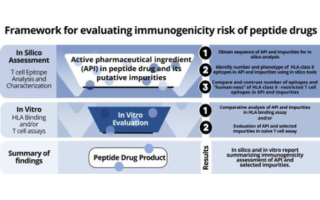
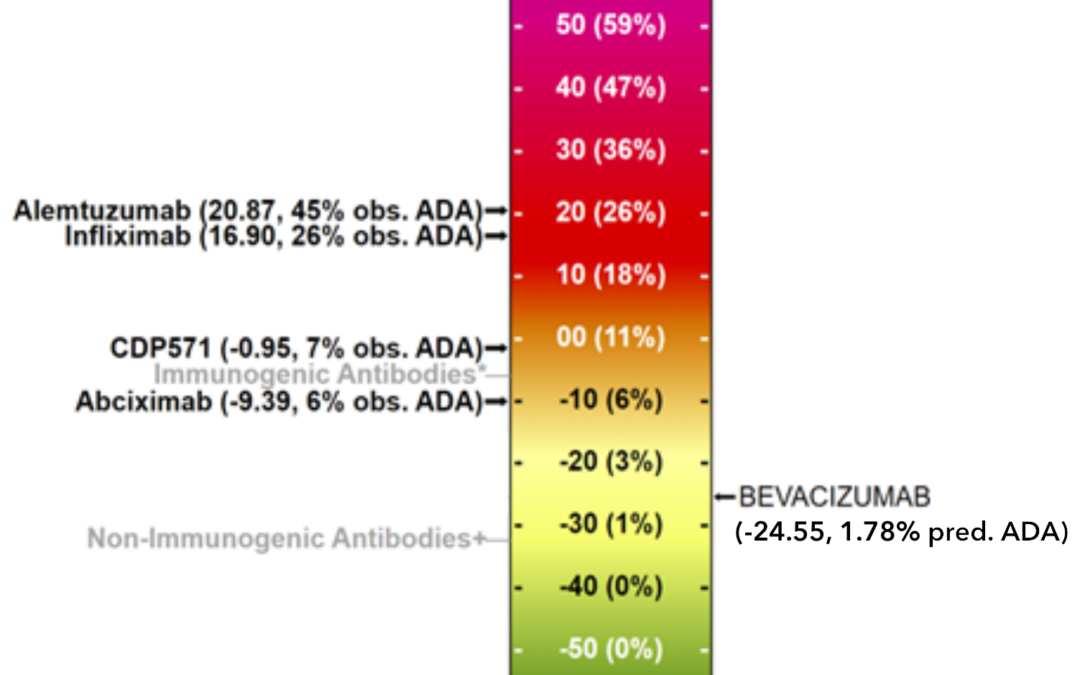
iVAX for antigen discovery, vaccine design, diagnostics, and epidemiology
iVAX for antigen discovery, vaccine design, diagnostics, and epidemiology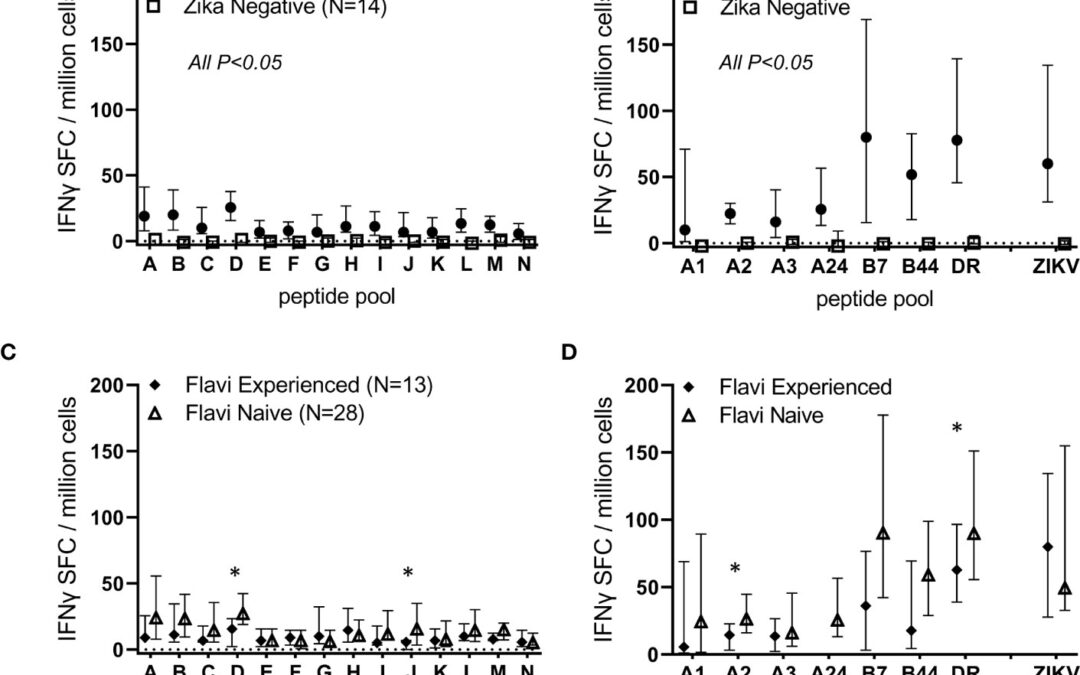
Identification of immunodominant T cell epitopes induced by natural Zika virus infection