Prevent immunogenicity surprises in the clinic.
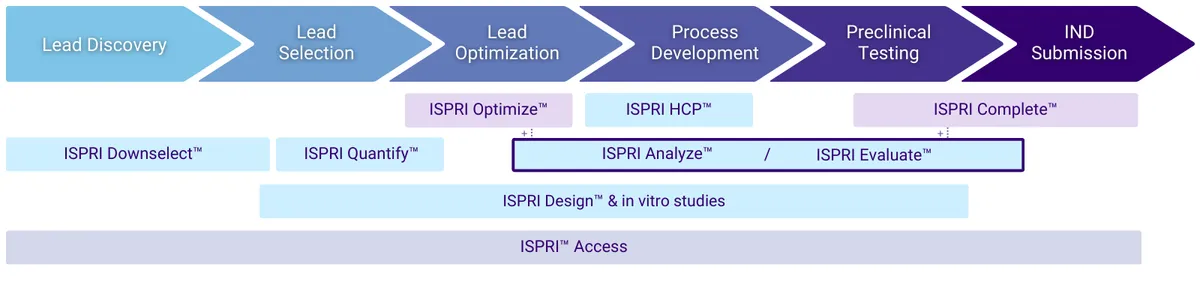
The ISPRI Evaluate™ report is a comprehensive evaluation of the immunogenic potential of a biologic therapeutic, leveraging EpiVax’s proprietary ISPRI™ in silico toolkit and key insights from immunogenicity experts.
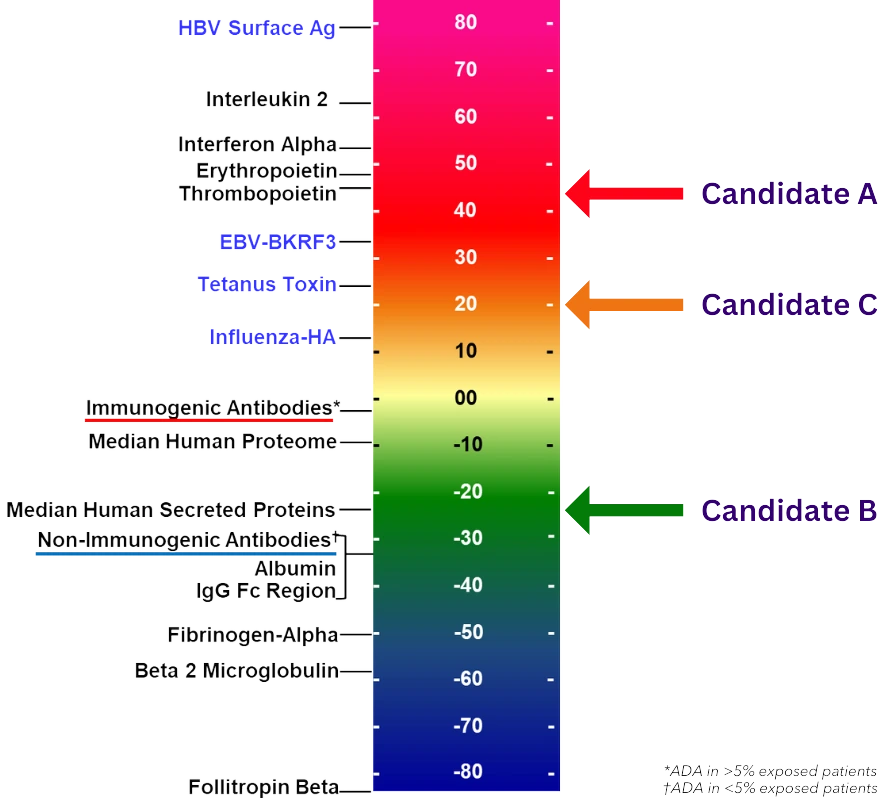
Beginning with a detailed review of your product’s primary amino acid sequence, this analysis delves into the candidate’s overall and regional immunogenic potential through the context of T cell epitope content and regulatory T cell epitope characterization. Regional ‘hotspots’ are identified, and overall risk is compared to similar products with known immunogenicity for benchmarking.
The final report will serve multiple purposes throughout the development process, combining objective measures with expert opinions crucial for directing the next phases of R&D.
Modalities
What can ISPRI Evaluate™ do for you?
-
Provide comprehensive immunogenicity data to include in:
– Integrated Summary of Immunogenicity (IND-filing preparations)
– Fundraising materials
– Publications
– Research grant applications
– Internal product characterization/R&D strategy discussions
-
Determine the overall and regional immunogenic potential of your lead candidate(s).
-
Rank order lead candidates allowing for intelligent selection and promotion.
-
Direct R&D strategies by reducing volume of downstream in vitro and in vivo immunogenicity studies, saving precious resources (typically 20-fold).
-
Focus optimization on critical protein regions.
ISPRI Analyze™
ISPRI Complete™
ISPRI Optimize™
If a full written report isn’t necessary, opt for the same detailed in silico analysis covering protein-level scores and regional immunogenic potential of a lead candidate (and a small set of variants) with this comprehensive data compilation, delivered in presentation form rather than a written report.
With this optional add-on to ISPRI Evaluate™, recieve a higher level of assessment in which EpiVax experts consider key clinical risk factors that could potentially influence a sequence’s ultimately observed immunogenicity, delivered in a written report with all findings interpreted and put into clinical context.
Product factors
- Sequence origin
- Mechanism of action
- Risk of aggregate formation
Treatment factors
- Dose frequency
- Route of administration
Patient factors
- Immune status
- Comedications
Select this optional add-on to ISPRI Evaluate™, and the EpiVax experts will make recommendations on which potentially immunogenic regions of your sequence could be good targets for deimmunization and offer a list of suggested point mutations to complete optimization.