Streamline R&D with actionable immunogenicity data.
Now offering a solution designed for monoclonal antibody developers seeking an even easier and faster immunogenicity risk assessment to characterize lead candidates! ISPRI Quantify™ provides a profile of immunogenic risk for a lead candidate with results that can guide your R&D strategy, allowing you to save time and money on downstream in vitro and in vivo immunogenicity studies.
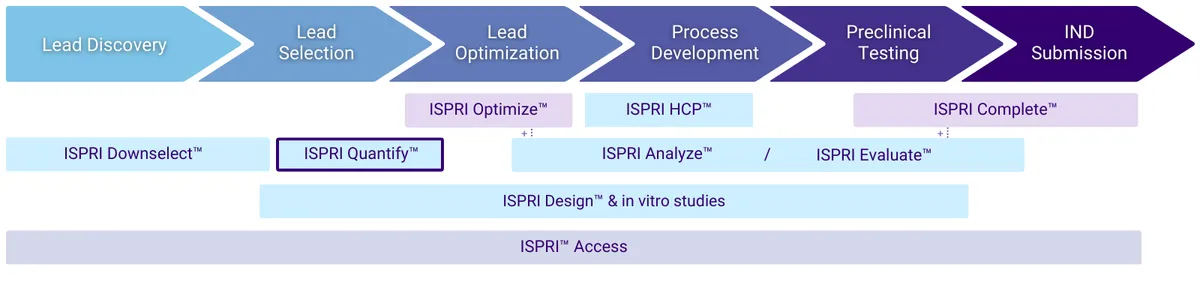
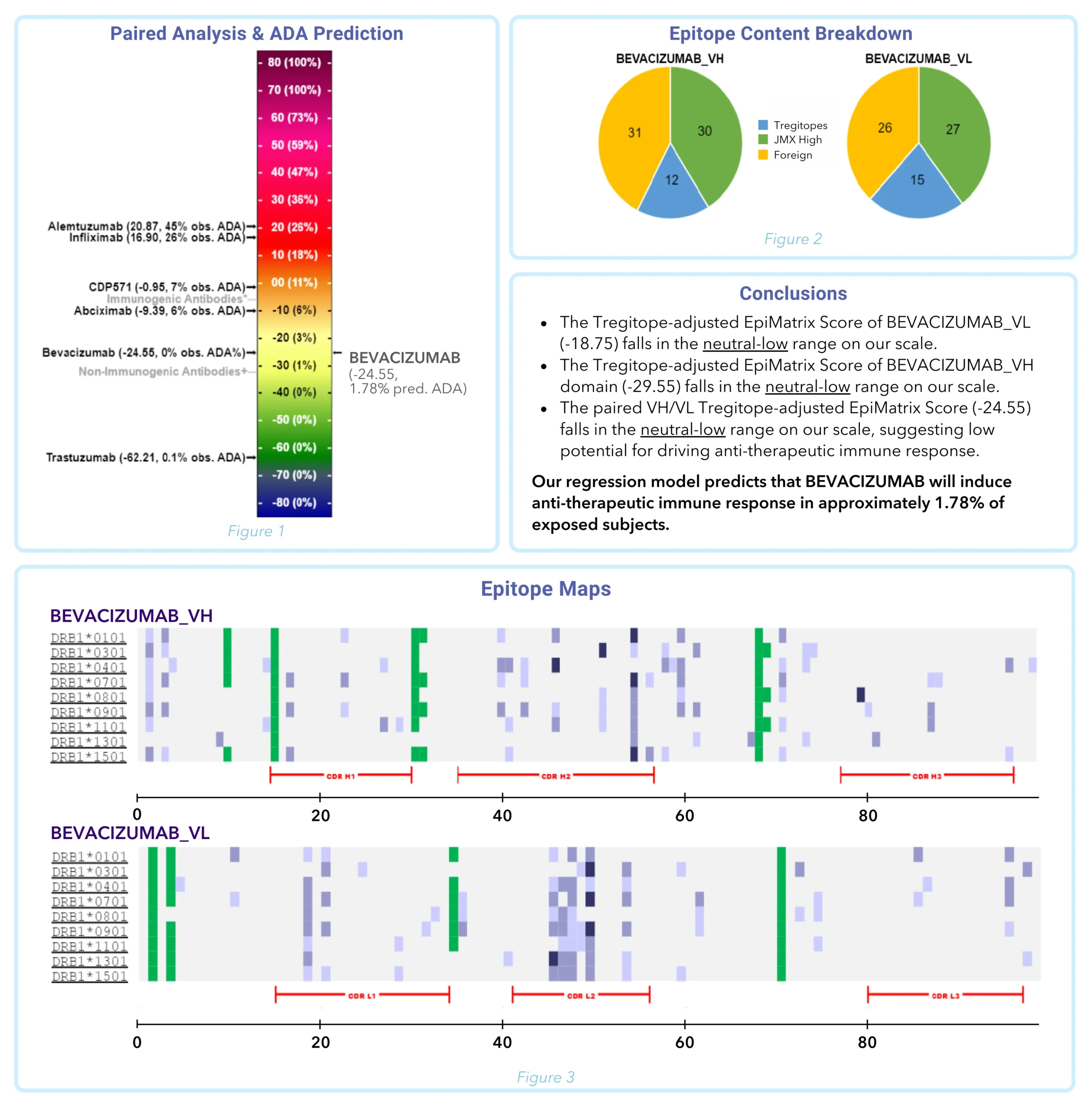
With this “just the facts” analysis, you’ll receive a rapid, precise, and holistic immunogenicity overview. The assessment compiles what EpiVax experts consider to be the most critical scores available from our in silico toolkit to enable an accurate prediction of immunogenic potential, including details on sequence-level T cell epitope and “humanness” scoring. These scores are ranked against mAbs of known immunogenicity for easy benchmarking (Figure 1), a high-level “T cell epitope content breakdown” (Figure 2) is provided, as well as T cell epitope maps to give regional immunogenicity insights (Figure 3) to classify the number of regulatory, potentially tolerated, and foreign epitopes in your sequence (key considerations for the most accurate risk assessment).
For a deeper clinical perspective, clients may opt for Quantify Plus™, which adds additional context by considering the influence of product, patient, and treatment-related factors on immunogenicity. This enhanced assessment provides a more holistic view of immunogenicity risk to better guide your immunogenicity risk mitigation strategy as you move forward in development.
ISPRI Quantify™ will empower you to navigate the complexities of immunogenicity risk swiftly and confidently.
Who is it for?
What can ISPRI Quantify™ do for you?
Provide immunogenicity data to include in:
Internal product characterization / R&D strategy discussions
Fundraising materials
Publications
Research grant applications
Determine the overall and regional immunogenic potential of your lead candidate(s).
Direct R&D strategies by reducing volume of downstream in vitro and in vivo immunogenicity studies, saving precious resources (typically 20-fold).
Gain insights on potential sequence optimization needs.
Sample ISPRI Quantify™ Report:
Quantify Plus™
For a deeper clinical perspective that enhances understanding of how product, patient, and treatment-related factors might influence immunogenicity, opt for Quantify Plus™. This enhanced assessment, conducted by EpiVax immunology experts, incorporates critical elements such as intended dosage, route of administration, formulation factors, and interactions with the patient’s immune profile, delivering a more comprehensive view of potential immunogenicity risk.
Need actionable immunogenicity data?
Learn more about ISPRI Quantify™!
Need a more comprehensive immunogenicity report? Check out ISPRI Evaluate™!