PROVIDENCE, RI, November 30, 2021 /PRNewswire/ — Today EpiVax, Inc. (“EpiVax”) confirmed that the company’s EPV-CoV-19 vaccine epitope sequences are 98.2% conserved in the new Omicron SARS-CoV-2 variant. EpiVax and EpiVax Therapeutics, Inc. (“EVT”) are also affirming commitment to move the novel T cell epitope vaccine into the clinic in 2022. EPV-CoV-19, is intended for use to protect against variants of concern (VOC) after primary SARS-CoV-2 exposure or vaccination. The vaccine is nearing the clinical phase of development while EpiVax and EVT team members finalize formulation, IND-filing, and the clinical trial protocol.
The EPV-CoV-19 vaccine will boost T cell response, a key contributor to immune protection against SARS-CoV-2 [References below]. Using proprietary immunoinformatics tools (described in Meyers et al. and De Groot et al.), EpiVax selected highly immunogenic sequences (T cell epitopes) conserved across all new types of SARS-CoV-2, including alpha, beta, gamma, delta and omicron. Today’s analysis (see figure below) of 188,758 SARS-CoV-2 sequences revealed that the vaccine epitopes are 97-99% conserved against VOC. Despite the very high number of mutations in the Omicron VOC, only three of 164 vaccine epitopes are expected to be ineffective in protecting against Omicron VOC.
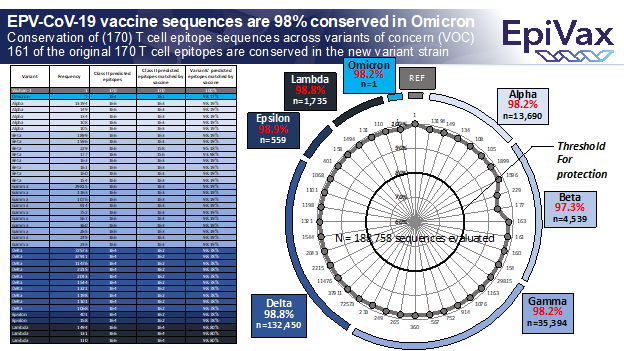
Most COVID vaccine developers have focused on antibody response to the pandemic virus while EpiVax experts, who lead the world in computational vaccine design, have steadfastly observed that vaccine-induced T cell responses play a significant role in protection afforded by exposure and vaccination. EpiVax focused on identifying the highest quality (highly immunogenic) T cell epitopes that are cross-conserved across coronavirus strains, to create a vaccine likely to be effective against all SARS-CoV-2 variants identified to date or emerging soon, including Omicron. The vaccine epitopes were shown to be effective in human in vitro and murine in vivo studies [Meyers et al, 2021]. EPV-CoV-19 has been licensed to EVT, a privately held, investor-backed company also located in Rhode Island.
About EpiVax:
EpiVax is a biotechnology company with expertise in T cell epitope prediction, immune modulation, and rapid vaccine design. EpiVax’s immunogenicity screening toolkits for therapeutics and vaccines, ISPRI and iVAX, are employed in advancing the research of a global roster of companies.
For more information about EpiVax, visit www.epivax.com.
About EVT:
EVT, founded in 2017, employs world-leading technology from EpiVax to activate T cells to cure or prevent disease. EVT’s pipeline includes a COVID-19 vaccine and a personalized bladder cancer vaccine. For more information about plans for EPV-COV-19, visit this link, or contact Nicole Ruggiero, COO of EVT.
For more information about EVT, visit www.epivaxtx.com.
Press Contact:
Katie Porter, Business Development Manager
EpiVax
Source: EpiVax, Inc.
References:
1. Sette, A. & Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 184, 861–880 (2021).
2. Huang, A. T. et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat. Commun. 11, 4704 (2020).
3. Choe, P. G. et al. Waning antibody responses in asymptomatic and symptomatic SARS-CoV-2 infection. Emerg. Infect. Dis. 27, 327–329 (2021).
4. Perreault, J. et al. Waning of SARS-CoV-2 RBD antibodies in longitudinal convalescent plasma samples within four months after symptom onset. Blood. https://doi.org/10.1182/blood.2020008367 (2020).
5. Ogega, C. O. et al. Durable SARS-CoV-2 B cell immunity after mild or severe disease. J. Clin. Invest. https://doi.org/10.1172/jci145516 (2021).
6. Okba, N. M. A. et al. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg. Infect. Dis. 26, 1478–1488 (2020).
7. Meyers, L.M., Gutiérrez, A.H., Boyle, C.M. et al. Highly conserved, non-human-like, and cross-reactive SARS-CoV-2 T cell epitopes for COVID-19 vaccine design and validation. npj Vaccines 6, 71 (2021). https://doi.org/10.1038/s41541-021-00331-6
8. De Groot AS, Moise L, Terry F, Gutierrez AH, Hindocha P, Richard G, Hoft DF, Ross TM, Noe AR, Takahashi Y, Kotraiah V, Silk SE, Nielsen CM, Minassian AM, Ashfield R, Ardito M, Draper SJ, Martin WD. Better Epitope Discovery, Precision Immune Engineering, and Accelerated Vaccine Design Using Immunoinformatics Tools. Front Immunol. 2020 Apr 7;11:442. doi: 10.3389/fimmu.2020.00442. PMID: 32318055; PMCID: PMC7154102.


