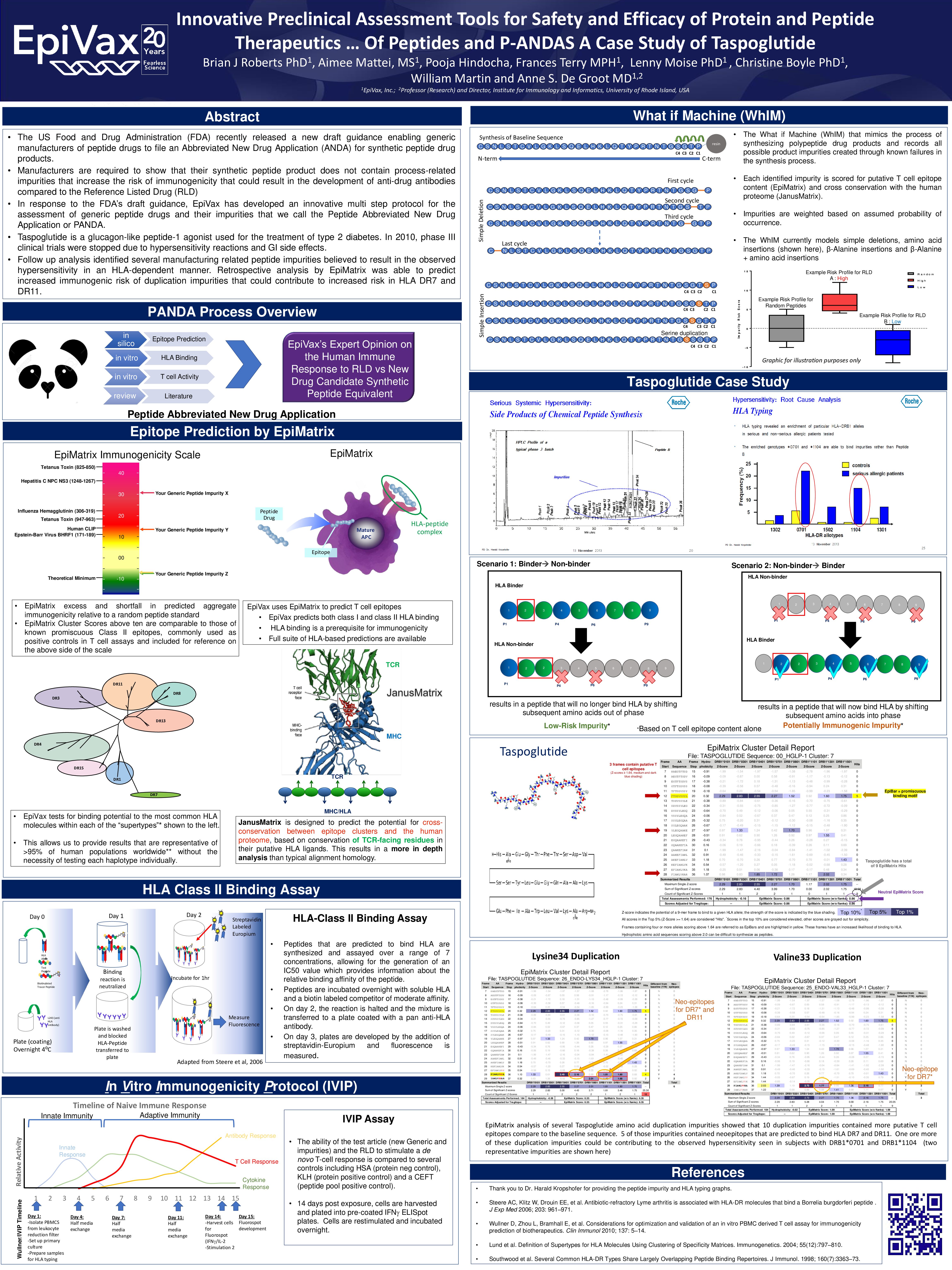


Better Epitope Discovery, Precision Immune Engineering, and Accelerated Vaccine Design Using Immunoinformatics Tools
Better Epitope Discovery, De Groot et.al. 2020
T cell epitope content comparison (EpiCC) analysis demonstrates a bivalent PCV2 vaccine has greater T cell epitope overlap with field strains than monovalent PCV2 vaccines
2020 Bandrick et al. EpiCC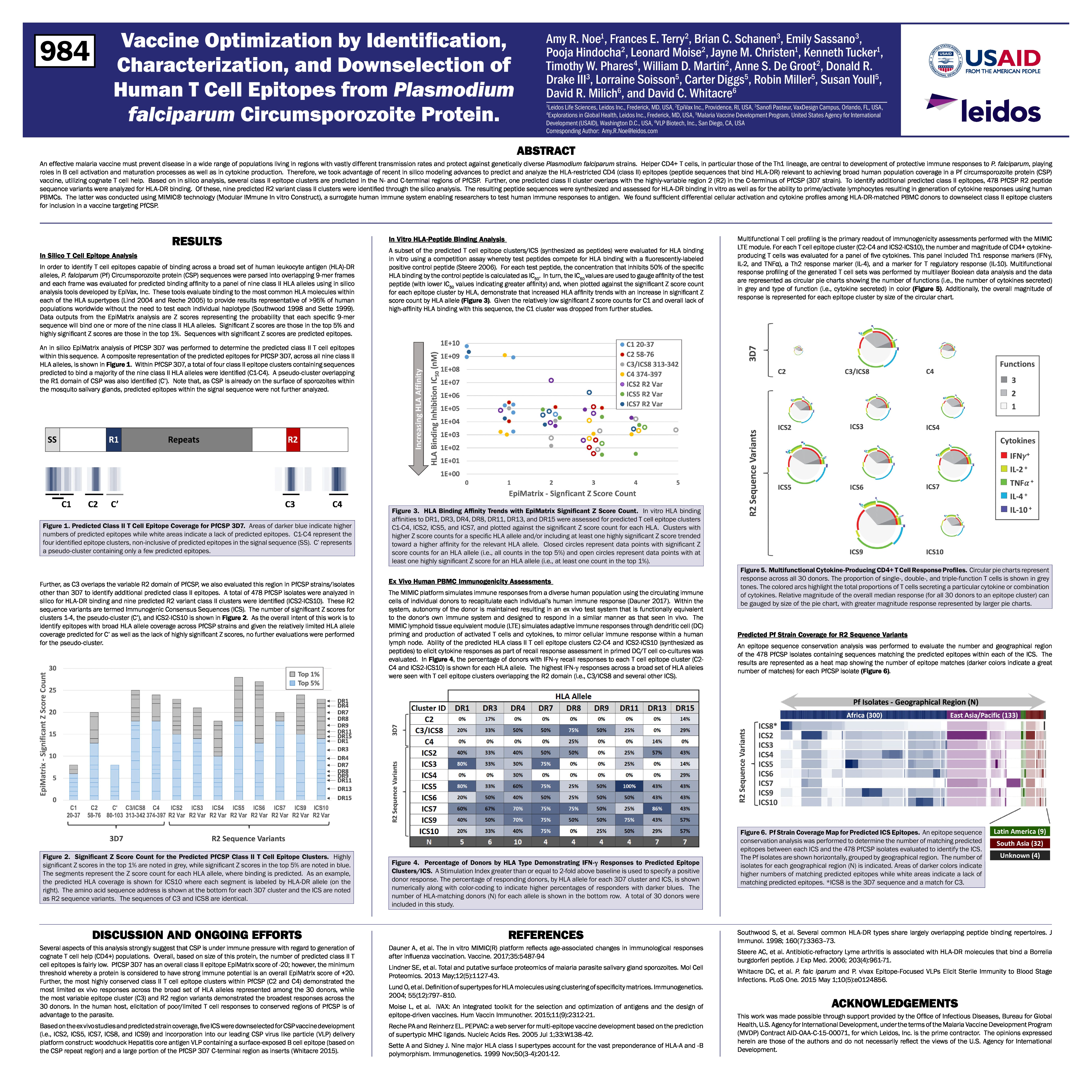
Vaccine Optimization by Identification, Characterization, and Downselection of Human T Cell Epitopes from Plasmodium falciparum Circumsporozoite Protein.
Vaccine Optimization by Identification, Characterization, and Downselection of Human T Cell Epitopes from Plasmodium falciparum Circumsporozoite Protein.