Shah Md. Shahjahan Miah, Andres Gutierrez, Sandra Lelias, Christine M. Boyle, Lenny Moise, Anne S. De Groot
EpiVax Inc, Providence, RI USA
Center for Vaccines and Immunology, University of Georgia, Athens, GA USA
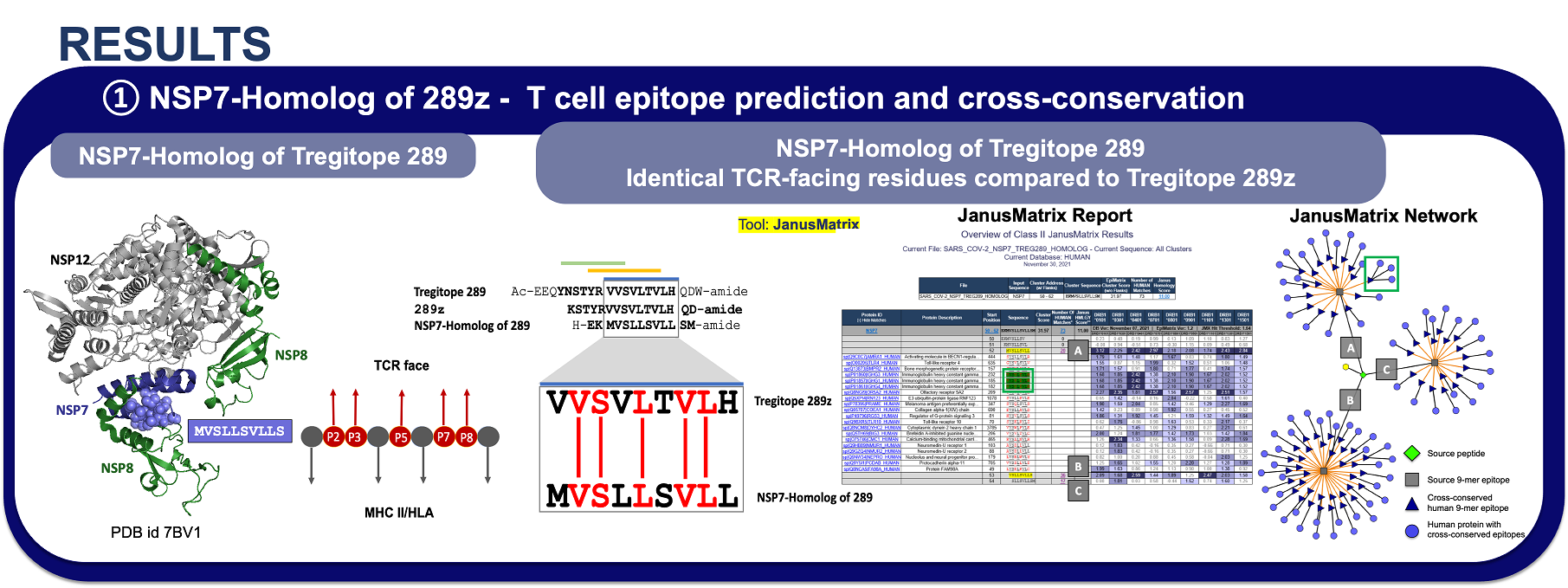
Abstract: Pathogens can escape host defenses by interference with innate immune mechanisms, B-cell and T-cell epitope deletion and taking on human appearances, also known as immune camouflage. Using the interactive vaccine design computational platform iVAX, we discovered a highly conserved, human-like T cell epitope in non-structural protein 7 (NSP7) of SARS-COV-2, which is a critical part of the RNA-dependent RNA polymerase hetero-tetramer complex, which is comprised by NSP7, two NSP8 molecules and the catalytic NSP12 required for coronavirus RNA synthesis. Remarkably, the epitope bears homology to a highly conserved regulatory T cell epitope (Tregitope) found in immunoglobulin G (IgG) Fc region (Tregitope 289). Based on observations for Tregitope 289, we hypothesized that the SARS-COV-2 NSP7 sequence may induce suppressive responses by activating regulatory T cells. We had previously demonstrated that Tregitope 289 is conserved across mammalian species and binds to HLA DR that cover >90% of the human population. Tregitope 289 stimulates a regulatory T cell phenotype (CD4+CD25+FoxP3+), and when co-delivered with antigenic sequences and other IgG Tregitopes, it induces antigen-specific tolerance. We tested both in vitro and found that Tregitope 289, the NSP7 epitope binds across multiple HLA-DRB1 supertype alleles in in vitro HLA class II binding assay. We also validated the suppressive capacity of the NSP7 epitope in our bystander suppression assays for CD4 T and CD8 T memory responses using PBMCs from an HLA-DRB1-diverse cohort. Identification and in vitro validation of the immunosuppressive NSP7 epitope of SARS-COV-2 may provide insight into the mechanisms by which pathogens can evade immune response and provide insight into the role of Tregs in COVID-19, which remains to be determined. In addition, it may permit researchers to better design variant-proof SARS-COV-2 and pan beta-coronavirus vaccines by engineering out pathogen-encoded human-like sequences that suppress immune response.
