
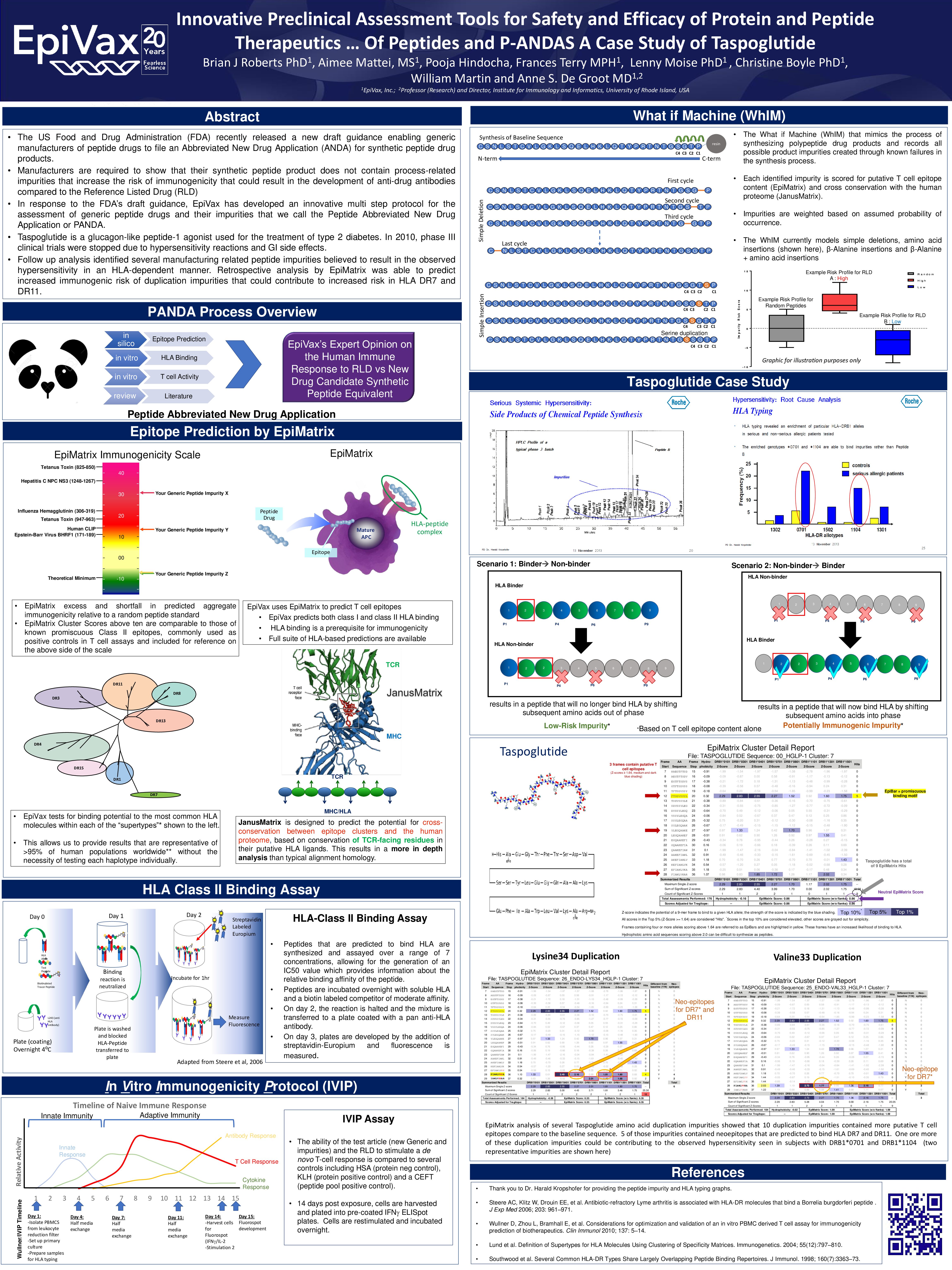
Innovative Preclinical Assessment Tools for Safety and Efficacy of Protein and Peptide Therapeutics … Of Peptides and P-ANDAS A Case Study of Taspoglutide
EpiVax_PANDA_Taspoglutide_April_19_Final
Beyond humanization and de-immunization: tolerization as a method for reducing the immunogenicity of biologics