PROVIDENCE, RI, November 4, 2021 /PRNewswire/ — EpiVax, Inc. (“EpiVax”) is pleased to announce an important commercial licensing agreement with Maruho Co., Ltd. (“Maruho”) for EpiVax’s patented Tregitope technology. This marks an exciting step for Maruho and EpiVax towards continued expansion of the Tregitope program and translation to the clinic.
Tregitopes are immune-modulating peptides derived from human immunoglobulin. Tregitopes were first discovered in 2008 by EpiVax co-founders Dr. Anne De Groot and William Martin. EpiVax has since validated many more Tregitopes and has developed a strong Tregitope patent estate. With a wide range of therapeutic applications, Tregitopes can be co-formulated or fused to proteins to induce antigen-specific tolerance. EpiVax is currently licensing Tregitope for additional indications with commercial and academic partners. More information on the EpiVax Tregitope Platform is available here or click the Q&A document below.
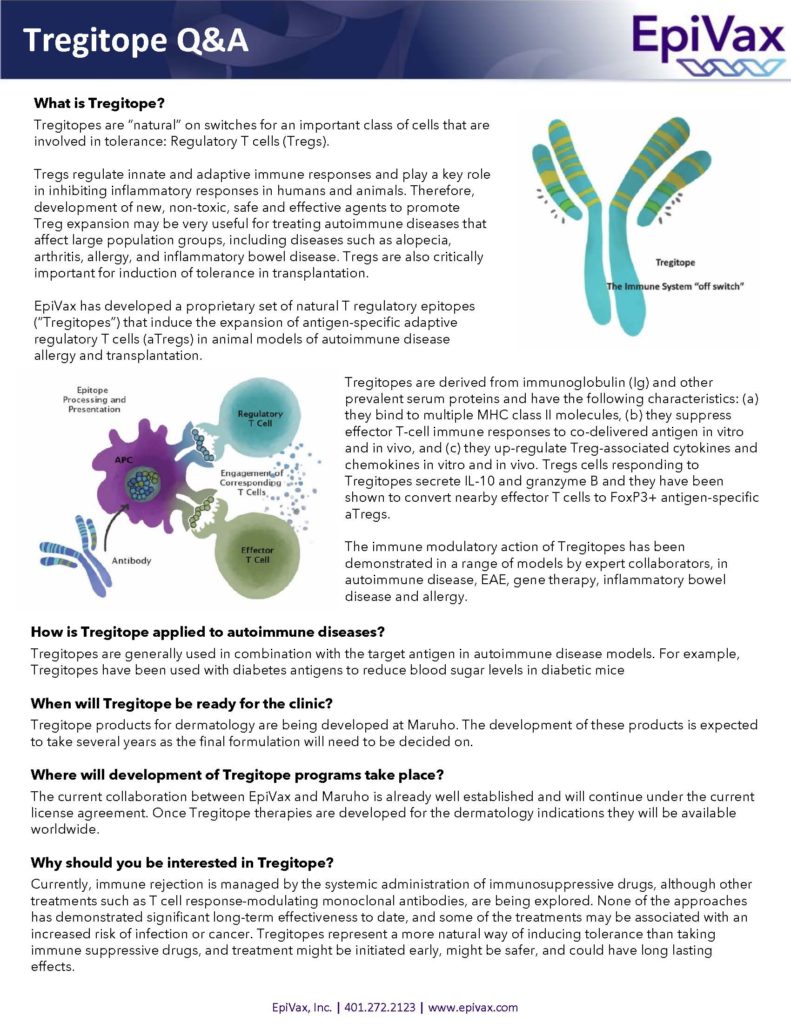 Similar to intravenous immunoglobulin G (IVIG) therapy, Tregitopes engage regulatory T cells and downregulate inflammation in a wide range of disease models. This mechanism of action has been studied in academic collaborations at McGill University, Harvard Medical School, and undisclosed commercial partners. There have been many peer-reviewed publications on the Tregitope platform in recent years. A full list can be found here.
Maruho will be able to leverage their Tregitope license to enhance their in-house therapeutic development strategies for autoimmune conditions. Annie De Groot MD, CEO/CSO of EpiVax, said “This agreement reflects the potential for Tregitopes, natural Treg epitopes that help regulate immune responses, to be used for the treatment of conditions that afflict patients around the world”.
About EpiVax:
EpiVax is a biotechnology company with expertise in T cell epitope prediction, immune modulation, and rapid vaccine design. EpiVax’s immunogenicity screening toolkits for therapeutics and vaccines, ISPRI and iVAX, are employed in advancing the research of a global roster of companies.
For more information about EpiVax, visit www.epivax.com.
About Maruho Co., Ltd.:
Maruho Co., Ltd. has its headquarters in Osaka and leads Japan in research and development, manufacturing and commercialization of dermatological products. Maruho is striving to improve the health and quality of life of people all over the world.
For more information, please visit https://www.maruho.co.jp/english/
For more information on Tregitope and licensing opportunities please contact us here.
Press Contact:
Katie Porter, Business Development Manager
EpiVax
[email protected]
Source: EpiVax, Inc.
Similar to intravenous immunoglobulin G (IVIG) therapy, Tregitopes engage regulatory T cells and downregulate inflammation in a wide range of disease models. This mechanism of action has been studied in academic collaborations at McGill University, Harvard Medical School, and undisclosed commercial partners. There have been many peer-reviewed publications on the Tregitope platform in recent years. A full list can be found here.
Maruho will be able to leverage their Tregitope license to enhance their in-house therapeutic development strategies for autoimmune conditions. Annie De Groot MD, CEO/CSO of EpiVax, said “This agreement reflects the potential for Tregitopes, natural Treg epitopes that help regulate immune responses, to be used for the treatment of conditions that afflict patients around the world”.
About EpiVax:
EpiVax is a biotechnology company with expertise in T cell epitope prediction, immune modulation, and rapid vaccine design. EpiVax’s immunogenicity screening toolkits for therapeutics and vaccines, ISPRI and iVAX, are employed in advancing the research of a global roster of companies.
For more information about EpiVax, visit www.epivax.com.
About Maruho Co., Ltd.:
Maruho Co., Ltd. has its headquarters in Osaka and leads Japan in research and development, manufacturing and commercialization of dermatological products. Maruho is striving to improve the health and quality of life of people all over the world.
For more information, please visit https://www.maruho.co.jp/english/
For more information on Tregitope and licensing opportunities please contact us here.
Press Contact:
Katie Porter, Business Development Manager
EpiVax
[email protected]
Source: EpiVax, Inc.
 Similar to intravenous immunoglobulin G (IVIG) therapy, Tregitopes engage regulatory T cells and downregulate inflammation in a wide range of disease models. This mechanism of action has been studied in academic collaborations at McGill University, Harvard Medical School, and undisclosed commercial partners. There have been many peer-reviewed publications on the Tregitope platform in recent years. A full list can be found here.
Maruho will be able to leverage their Tregitope license to enhance their in-house therapeutic development strategies for autoimmune conditions. Annie De Groot MD, CEO/CSO of EpiVax, said “This agreement reflects the potential for Tregitopes, natural Treg epitopes that help regulate immune responses, to be used for the treatment of conditions that afflict patients around the world”.
About EpiVax:
EpiVax is a biotechnology company with expertise in T cell epitope prediction, immune modulation, and rapid vaccine design. EpiVax’s immunogenicity screening toolkits for therapeutics and vaccines, ISPRI and iVAX, are employed in advancing the research of a global roster of companies.
For more information about EpiVax, visit www.epivax.com.
About Maruho Co., Ltd.:
Maruho Co., Ltd. has its headquarters in Osaka and leads Japan in research and development, manufacturing and commercialization of dermatological products. Maruho is striving to improve the health and quality of life of people all over the world.
For more information, please visit https://www.maruho.co.jp/english/
For more information on Tregitope and licensing opportunities please contact us here.
Press Contact:
Katie Porter, Business Development Manager
EpiVax
[email protected]
Source: EpiVax, Inc.
Similar to intravenous immunoglobulin G (IVIG) therapy, Tregitopes engage regulatory T cells and downregulate inflammation in a wide range of disease models. This mechanism of action has been studied in academic collaborations at McGill University, Harvard Medical School, and undisclosed commercial partners. There have been many peer-reviewed publications on the Tregitope platform in recent years. A full list can be found here.
Maruho will be able to leverage their Tregitope license to enhance their in-house therapeutic development strategies for autoimmune conditions. Annie De Groot MD, CEO/CSO of EpiVax, said “This agreement reflects the potential for Tregitopes, natural Treg epitopes that help regulate immune responses, to be used for the treatment of conditions that afflict patients around the world”.
About EpiVax:
EpiVax is a biotechnology company with expertise in T cell epitope prediction, immune modulation, and rapid vaccine design. EpiVax’s immunogenicity screening toolkits for therapeutics and vaccines, ISPRI and iVAX, are employed in advancing the research of a global roster of companies.
For more information about EpiVax, visit www.epivax.com.
About Maruho Co., Ltd.:
Maruho Co., Ltd. has its headquarters in Osaka and leads Japan in research and development, manufacturing and commercialization of dermatological products. Maruho is striving to improve the health and quality of life of people all over the world.
For more information, please visit https://www.maruho.co.jp/english/
For more information on Tregitope and licensing opportunities please contact us here.
Press Contact:
Katie Porter, Business Development Manager
EpiVax
[email protected]
Source: EpiVax, Inc.


