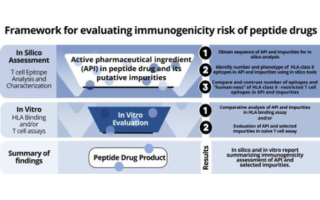


In silico immunogenicity assessment for sequences containing unnatural amino acids: A method using existing in silico algorithm infrastructure and a vision for future enhancements
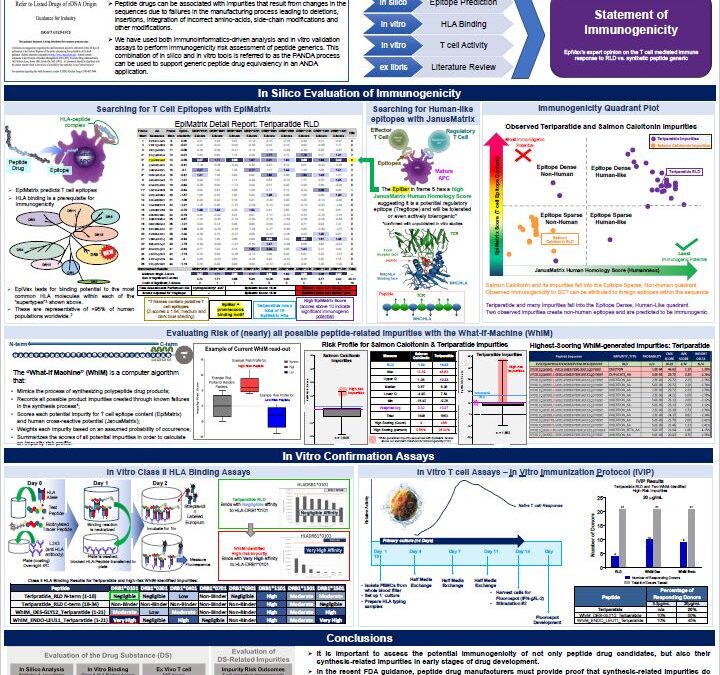
Poster: Innovative preclinical assessment tools for safety and efficacy of protein and peptide therapeutics… Of Peptides and P-ANDAS
Innovative Preclinical Assessment Tools for Safety and Efficacy of Protein and Peptide Therapeutics… of Peptides and P-ANDAS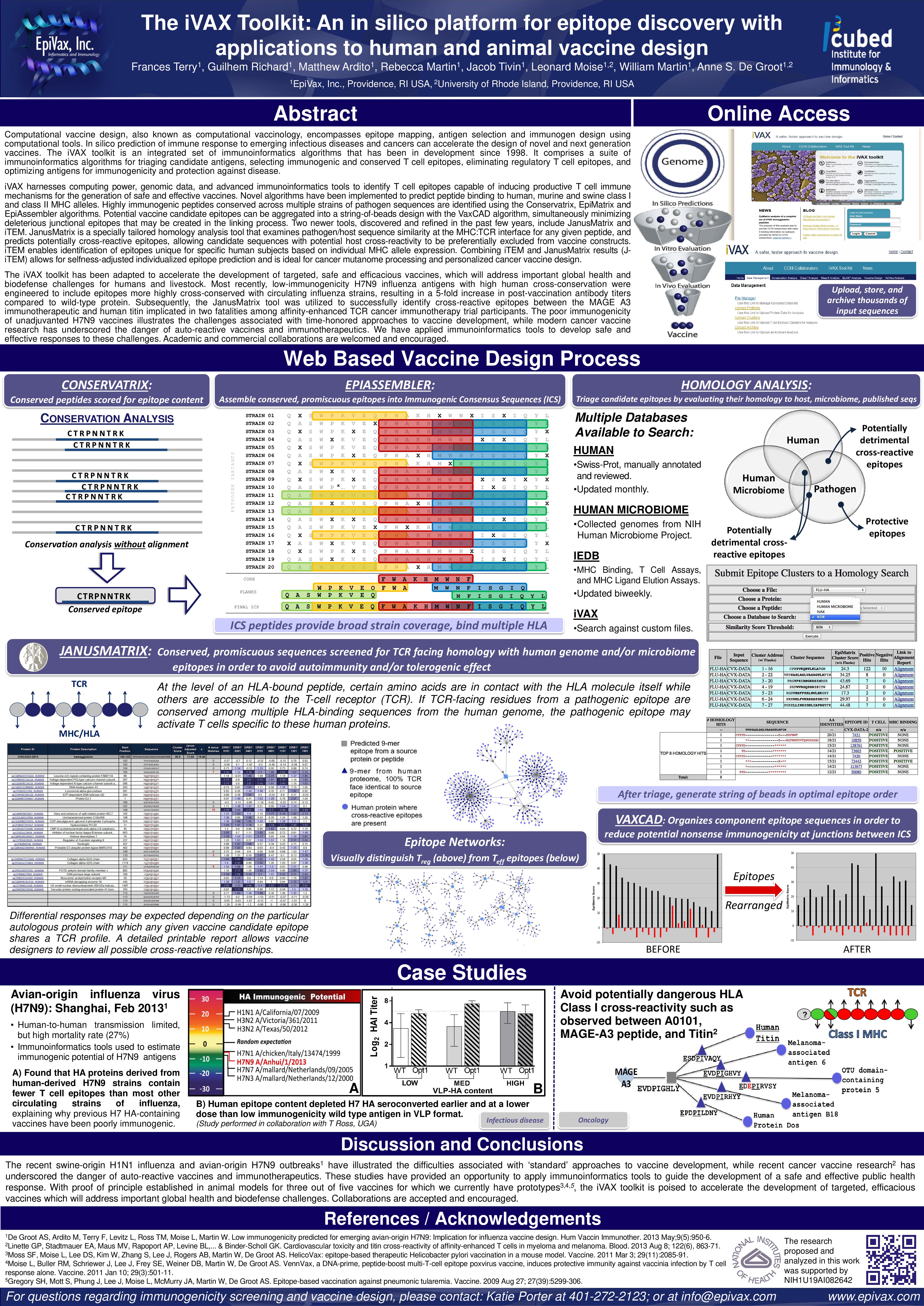
The iVAX Toolkit: An in silico platform for epitope discovery with applications to human and animal vaccine design
EpiVax_ISV_iVAX_23Oct19