Deimmunization Projects
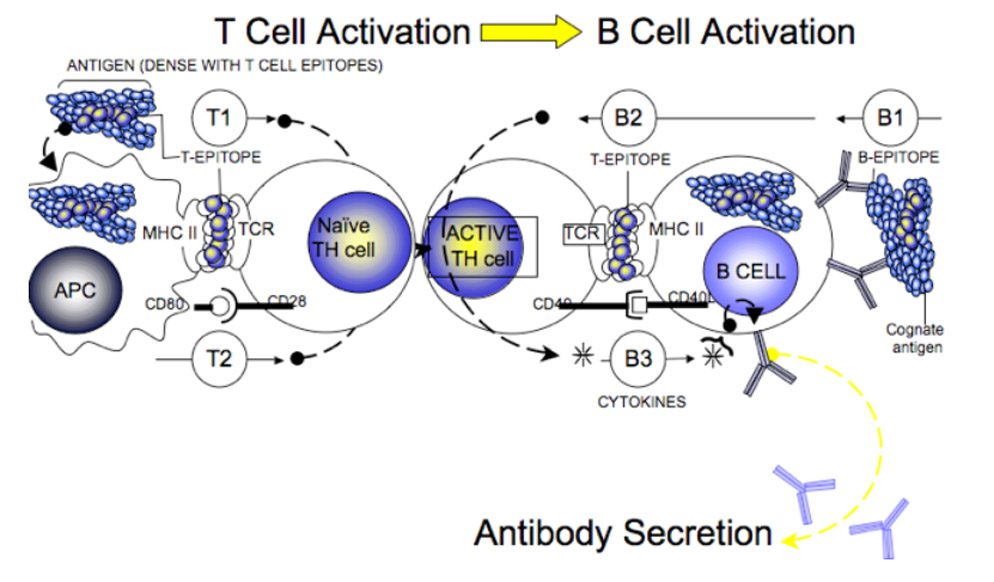
T-dependent B cell response is also absent.
Deimmunized Botox
Intramuscular injections of therapeutic botulinum neurotoxin type A (BoNT/A) have revolutionized treatment of movement disorders such as dystonia. Like many therapeutic proteins, BoNT/A is immunogenic and can lead to development of anti-drug antibodies – a major concern when treating chronic conditions that require repeated drug administrations. We are currently developing a less immunogenic version of BoNT/A through the process of T cell epitope modification known as deimmunization. A de-immunized version of BoNT/A would allow patients to experience the benefits of BoNT/A injections without developing an immune response against the treatment.
Deimmunized FVIII
Hemophilia A patients are prone to develop inhibitory immune responses to the very therapy they require: Factor VIII protein replacement. FVIII appears foreign to their immune systems, and the mechanisms of tolerance that would normally suppress immune responses to FVIII, an autologous protein, are absent or reduced. We are evaluating “antigen-specific adaptive tolerance induction” (ASATI), a novel approach to immunotherapy, and its application to FVIII therapy. This project is currently partnered with Biotest AG.
![]()
