That’s right, what does it mean? When we heard that the FDA suspended two clinical trials of IB1001, a recombinant Factor IX protein produced in Chinese hamster ovary (CHO) cells in July 2012 due to development of antibodies to the host cell proteins, we were surprised that there wasn’t a lot more attention paid to the topic.  What’s even more interesting is that the immune responses among patients given the IB1001 drug were sufficient to make the FDA put a clinical hold on the trial. Something is going on here and we don’t have all the information we need.
What’s even more interesting is that the immune responses among patients given the IB1001 drug were sufficient to make the FDA put a clinical hold on the trial. Something is going on here and we don’t have all the information we need.
Why blog about it? Well, we think that immune response to HCP is a big deal. See my previous post about it here. Some people might think that anti-CHO antibodies are not a big deal, since after all, the antibodies to HCP don’t appear to affect the drug (Factor IX in this case). But, we ask you: would you want to make an immune response to a protein that is a lot like your own proteins? That’s right, we thought not. That kind of anti-host cell protein response is not one I would want in my blood, and not in my patients’ blood, thank you.
Immune responses to HCP may cross-react with “self” proteins
Why worry? Because immune responses to HCP may cross-react with “self” proteins. How do we know? Well, we can go back to studies that have been performed over the last thirty years or so by my dad. His name Is Leslie J. De Groot (here’s a recent webpage). He has been injecting human thyroid stimulating hormone receptor (HuTSHR) into mice to produce a model of Grave’s disease (thyroid disease) for, well, about the past three decades. The mice make antibodies to “self” after being injected with the slightly dissimilar human version of their protein (the analogy would be to humans getting injected with slightly dissimilar CHO proteins). And our friend and colleague David Scott uses human FVIII to make mice who are otherwise tolerant to their own mouse FVIII, intolerant (see our collaboration with him here). So, if you follow the argument, similar doses of CHO proteins could potentially cause immune responses to “self” in humans, and based on what we know about these animal models, the immune reactions could spread.
For a long time, people said – show us an example. Well now, Ipsen and Inspiration have produced one. The FDA was alerted after patients treated with the experimental product were found to be more likely to test positive for antibodies to the product’s host cell protein, CHO. Well we’re not here just to make noise about this, no, instead, we propose a solution.
A solution to CHO HCP immunogenicity
What’s the solution? Map the HCP for potential immunogenicity, using EpiMatrix and our Immunogenicity scale. See below for an example.
We plan to make a public database of CHO Proteins that have an “EpiMatrix score” (measure of epitope content) that is adjusted for sequence similarity to human homologs.
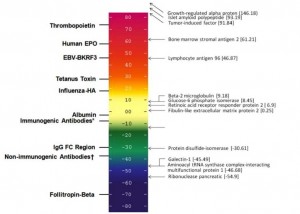
CHO Proteins are shown on our standard Immunogenicity Scale. Any score above 20 is potentially immunogenic. Determination of sequence deviation from human homolog would need to be factored into risk assessment.(see previous blog post for details). We plan to make this information available on line (CHO immunogenicity Look Up Table coming soon). We suggest that recombinant protein producers find the ideal “production run” that contains minimal amounts of low immunogenicity CHO proteins. That’s right, we can predict the potential for immunogenicity on our ISPRI immunogenicity scale – which for this purpose, will be adjusted for sequence overlap between Human and CHO.
That’s right, a public database of CHO protein immunogenicity. Stay tuned while we pull the resource together and put it on line. A first pass analysis is shown here, but we have been thinking it over; the outcomes may be adjusted (for homology), at least initially. We’ll be working hard over the next month to get the site up, to go along with our forthcoming publication. Yes, you can make lower HCP content proteins, and make sure that the proteins are all low immunogenicity before you administer to patients.
More information about normal human immune responses to HCP can be found here: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2811637/?tool=pubmed
Ipsen Says Hemophilia Drug Study Suspended by U.S.’s FDA
Inspiration Biopharmaceuticals Inc., a Cambridge-based company, produced the drug. Inspiration and Ipsen are testing their drug’s ability to prevent bleeding in adults with hemophilia B and in children who have received prior treatment for the illness. The FDA was alerted after patients treated with the experimental product were found to be more likely to test positive for antibodies to the product’s host cell protein, CHO.
Like other recombinant drugs such as monoclonal antibodies, IB1001 uses CHO cells to produce a protein key to coagulation known as factor IX. Those treated with the experimental medicine showed a higher-than-expected level of antibodies against the hamster cells, suggesting their immune systems had begun to mount a response to the treatment.
Although Ipsen has found no evidence suggesting a change in the drug’s current clinical benefit or risk profile, the suspension could hinder the company’s original plans to introduce the treatment in Europe and the US, or lead to its being dropped altogether. Inspiration was asked by the FDA to conduct a root cause analysis, the results of which will be known sometime this coming fall.
