
by Elena Iemma | Mar 29, 2024 | Featured, News
PROVIDENCE, RI, April 1, 2024 /PRNewswire/ — Read the publication. In the pursuit of developing novel biologic entities, scientists harnessing the power of AI and generative biology depend on computational tools for predicting and managing immunogenicity risk –...

by Elena Iemma | Jan 30, 2024 | Featured, Thinking Out Loud -Blog
It’s Post-Pandemic and we’re back on the road traveling to the 4 corners of the Earth. Where will we get to see you? Here’s a list of upcoming events, and because … what’s travel without great food? … I’ve included some...

by Elena Iemma | Dec 20, 2023 | Featured, News
PROVIDENCE, RI, December 20, 2023 /PRNewswire/ — EpiVax, Inc. (“EpiVax”), an internationally recognized leader in the field of immunogenicity, today announced that Eisai Co. Ltd. (“Eisai”) has licensed EpiVax’s ISPRI toolkit for immunogenicity risk assessment of...

by Elena Iemma | Nov 27, 2023 | Featured, Thinking Out Loud -Blog
This is a two-way street Hey Hey, Hallo, Hola, Ciao, Namaste, Hello, Bonjour, Konnichiwa! When do you do your ‘thinking’ about the day? Do you do it while running? Well, I was thinking about you guys on my runs this week. I am finally back in Providence,...
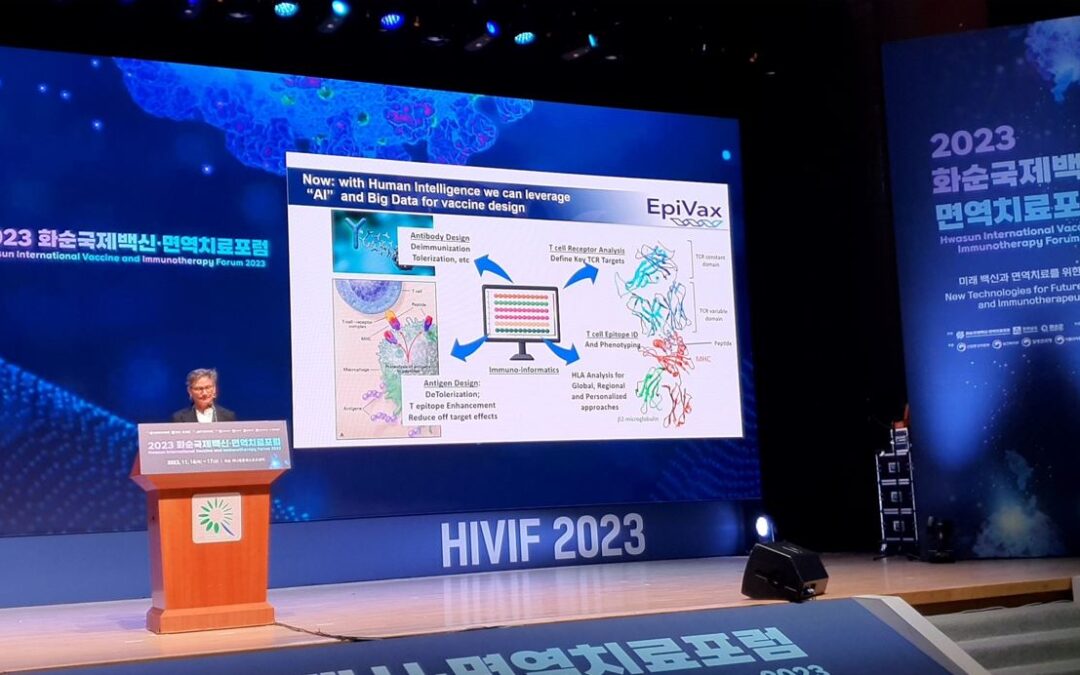
by Elena Iemma | Nov 20, 2023 | Featured, News
EpiVax CEO Annie De Groot was one of three plenary session speakers at a recent international forum on vaccines organized by Joon Rhee. Her topic was ‘Better, Safer, and Personalized Vaccines will Result from Human + Artificial Intelligence’. Other speakers...

by Elena Iemma | Aug 10, 2023 | Featured, News
PROVIDENCE, RI, August 10, 2023 /PRNewswire/ — EpiVax Inc. announces the publication of results from a research collaboration with the Food and Drug Administration aimed at streamlining generic drug evaluations. The research, initiated in 2018 with a $1M...







