The in silico tools here at EpiVax have been extensively validated, both internally and externally. That said, we always get a warm fuzzy feeling when we see our tools highlighted in papers and at conferences! Proud parents, we can’t help it😊
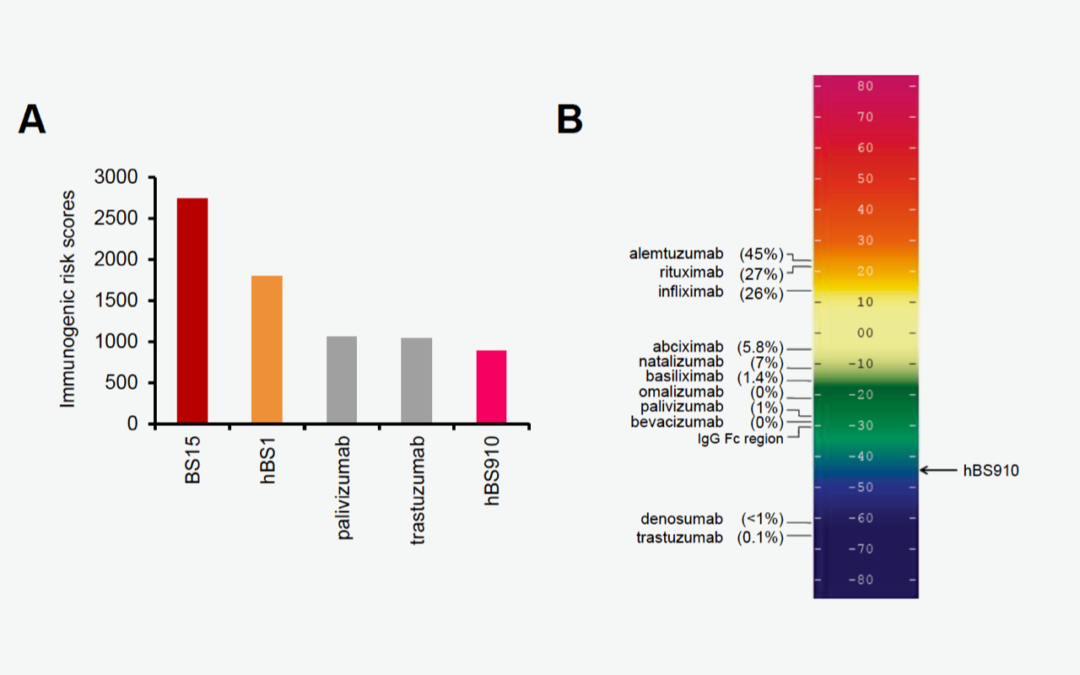
Just such a moment occurred last week when Valerie Quarmby (Genentech, a Roche subsidiary), presenting work by the innovative team of Zenjiro Sampei and Tom Igawa at Chugai Pharmaceutical (a Roche affiliate), reported that a “Complex mathematical formula” was used and showed this graphic during last week’s FDA Workshop on comparative immunogenicity risk assessment for generic peptide products.
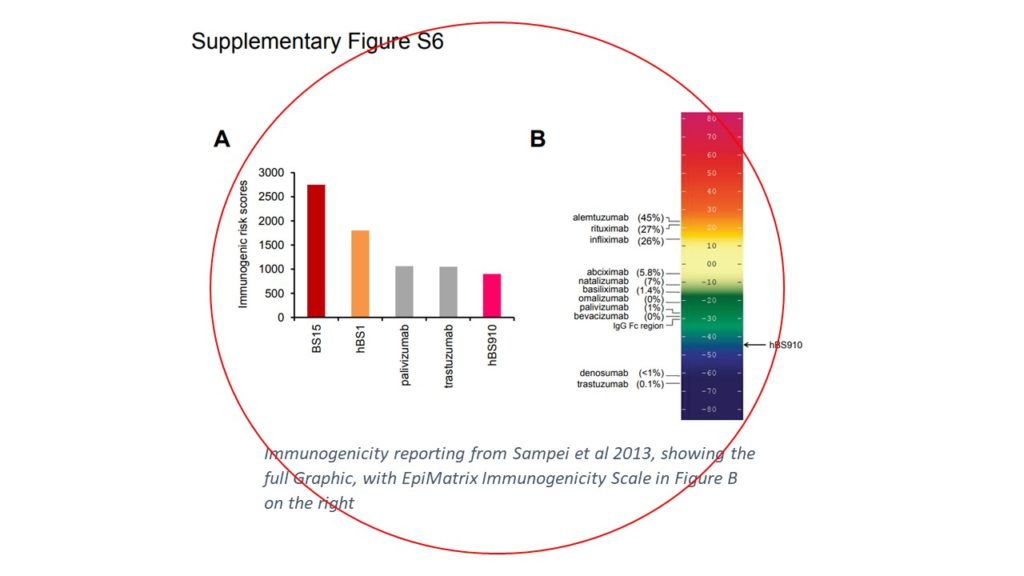
In short, the authors predicted immunogenicity potential as part of the antibody identification and optimization process. Researchers leveraged ISPRI and Tregitope-adjusted EpiMatrix scores, two EpiVax in silico tools. Subsequent T-cell assays confirmed the in silico predictions, and Chugai was off to the races for selecting their lead candidate.
Here is the publication link for those interested!
Interested in learning more about the EpiVax PANDA program for your generic peptide program? Check out our CEO Annie’s presentation at the FDA workshop here or reach out to us here to schedule a discussion.