In Vitro Immunization Protocol (IVIP)
T Cell Assays: Naïve Donors – In Vitro Immunization Protocol (IVIP)
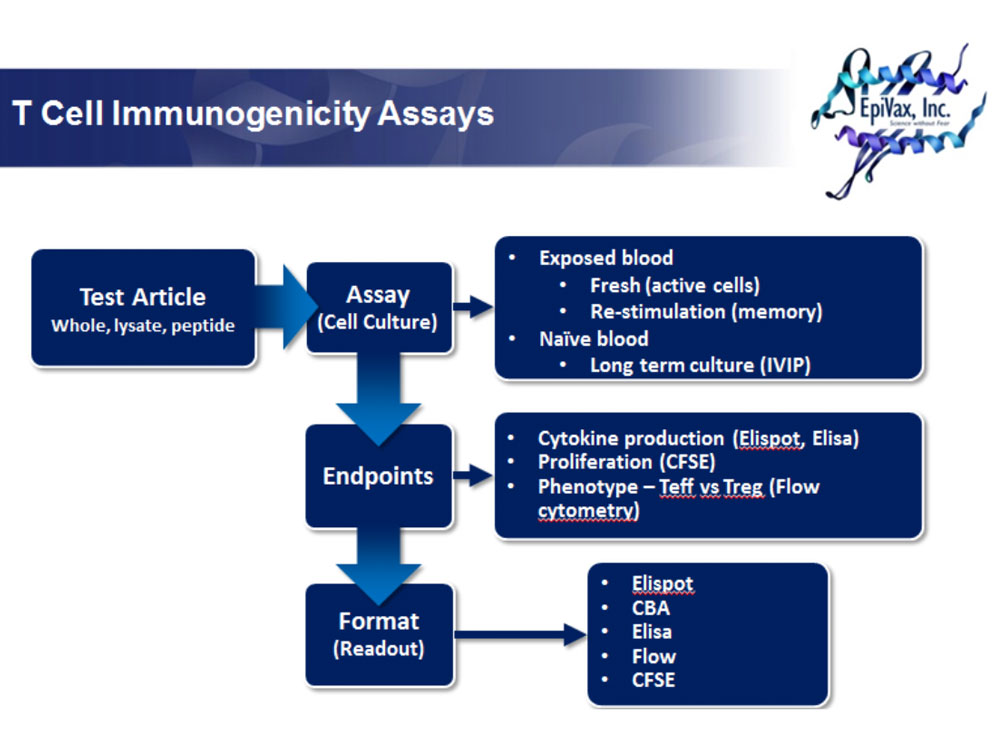
Naïve blood samples may be conditioned ex vivo through prolonged exposure to experimental antigen for predicting immunogenicity of therapeutic proteins. Compared to the recall response, the precursor frequency of antigen-specific cells in a naïve population is quite low. Thus, multiple rounds of antigen stimulation over several weeks is required to expand sufficient numbers of antigen-specific T cells for reliable measurement. Conditioning naive blood for assay is a difficult and time consuming process and, as such, we recommend starting this type of assay with a panel of at least ten subjects.

Researchers working in the labs at EpiVax in Providence. From left, Mitchell McAllister, research associate, Olivia Morin, research associate, working in their culture lab. PBN PHOTO/MICHAEL SALERNO
EpiVax has adapted an in vitro protocol from Wullner et. al [1] to test the immunogenicity of novel vaccines and therapeutics with human lymphocytes. Briefly, blood samples drawn from HLA-typed healthy subjects are cultured for 15 days. On days 2, 5, 9 and 12, each sample is stimulated with either whole protein or protein digest. On day 15, cultures are tested by ELISpot for IFNg and IL-5 responses. ELISpot plates are stimulated with whole protein, digested protein, peptide pools, and selected peptides. In addition, clients have the option of testing supernatants from ELISpots in multiplex ELISA for additional cytokines including IL-2, IL-6, IL-10, IL-17A, and TNFα, enabling finer characterization of responses as either Th1, Th2 or Th17. All experimental samples are tested beside relevant positive and negative controls.
T-Cell Assays: Exposed Donors
T cell assays can be utilized to identify and measure a recall or memory response in PBMC derived from subjects who have been exposed to a given biologic product either as therapy or within the context of a clinical trial. As the precursor frequency of the antigen specific cells in a recall response is relatively high, whole PBMC assays are both rapid and sensitive with no pre-treatment or enrichment required. In addition to recall responses with whole protein and digested protein, epitope-mapping can be performed using epitope specific peptides at the time of challenge.
Email Us Here for a PDF brochure describing our Lab-Based Services and/or a Cost Estimate for your project.
[1] Wullner D, Zhou L, Bramhall E, Kuck A, Goletz TJ, Swanson S, Chirmule N, Jawa V. Considerations for optimization and validation of an in vitro PBMC derived T cell assay for immunogenicity prediction of biotherapeutics. Clin Immunol. 2010 Oct;137(1):5-14.
![]()
