¡Hola! from Granada, Spain!
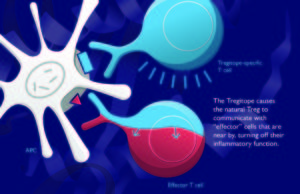
As it turns out, there are as many kinds of Tregs as there are types of tapas in Granada. But there is only one Tregitope. OK, make that several (in fact, there are six that we aim to use for Tregitope therapy in autoimmune disease). Meanwhile, in Granada, the copas are flowing, and Tregitope is being presented to the >3,000 participants at Autoimmunity 2012. Leslie Cousens, Ph.D. (EpiVax) and David W. Scott, Ph.D. (Vice Chair for Research, Department of Medicine at USUHS), presented “Tregitope mechanism of Action” and “IgG-Derived T Cell Epitopes (Tregitopes) Suppress Immune Responses by Activating nTregs”, respectively, today at 2 PM. David described the effect of Tregitopes in D011.10 FoxP3 transgenic mice (induction of iTregs) and Leslie described the three-step mechanism of action.
Tregitopes were also mentioned by Bruce Mazer, M.D. of McGill University during his talk on the effect of IVIG (induction of regulatory T cells) in asthma. Bruce has demonstrated that the induction of Tregs in asthmatic lungs is equal or better than Treg induction by IVIG, in the OVA-allergy model. Dr. Srini Kaveri Ph.D. (INSERM) has integrated Tregitopes into his presentation on the IVIG mechanism of action; that much was evident on Thursday.
In case you missed these talks, on Sunday, Annie De Groot M.D., EpiVax CEO, will present ” Tregitope applications to tolerance induction in autoimmune diseases” as the opening talk of the Plenary Session. Wassim Elyaman, Ph.D. is also presenting a poster on Tregitope application to Multiple Sclerosis. See here for a great picture of Wassim and Leslie at Wassim’s poster.
Tregitope was also featured at the May 2012 American Association of Immunologists (AAI) meeting, where poster presentations on the topic were “mobbed” according to eyewitnesses. Why the excitement? Because Tregitope truly represents a paradigm shift for autoimmunity and tolerance.
Stay tuned for more news on Tregitope – including Safety and Toxicity studies (scheduled for June, 2012) and proof-of-concept Phase I trials (to be scheduled in 2013). This is a banner year for Tregitopes – click our RSS feed to stay in the loop.