(This post has been corrected, thanks Sebastian!) I love “Google Alerts”. They let me know when someone has just published an article citing one of our articles, and link me to new, exciting work that has just been made available on the web. Last week, my google alert linked me to a new paper by Sebastian Spindeldreher’s group at Novartis: “Secukinumab, a novel anti-IL-17A antibody, shows low immunogenicity potential in human in vitro assays comparable to other marketed biotherapeutics with low clinical immunogenicity“. Wow, I thought, in vitro assays using peptides. Sorta old school, Novartis. Maybe they referenced our work because they did some in silico analysis too?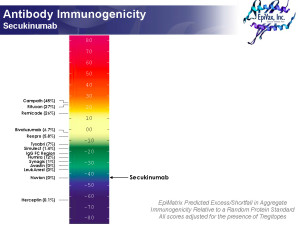
Nope. To my dismay, I found that they had conducted the evaluation by MAPPS, with no in silico screen. Hmmm, I thought, that’s really too bad, because had they done one, they would have saved themselves a lot of time and money (that is, if they had access to the ISPRI toolkit).
Actually, we predict Secukinumab to be low immunogenicity. In about 5 minutes. See picture. And Frances Terry, Director of Data Analysis at EpiVax, cranked that analysis out, over coffee. According to Frances:
Secukinumab is very low scoring (on ISPRI, using the Tregitope-adjusted immunogenicity scale (see picture) and is predicted to be non-immunogenic. Even though the heavy chain has a lot of epitope content, the vast majority of these epitopes correspond to Tregitopes (Treg epitopes identified by EpiVax in 2008). All four clusters in the heavy chain contain Tregitopes. Of two clusters in the light chain, one is a Tregitope and the other is in the CDR. All clusters are very human by JanusMatrix.
See? No sweat. 5 minutes. Over coffee. Wouldn’t you rather have that information in your back pocket before you spend weeks and weeks slaving over peptide assays? Not that we don’t do in vitro. But we definitely do in silico first. Wouldn’t you rather know where the epitopes might be, when you design your in vitro assays?
We hope that Novartis will join the “in the know” group – 9 out of 12 Big Pharma companies (and hundreds of “small to medium” biotech companies, like Chugai (a Roche company) that use the ISPRI toolkit. For more information, write me here.
