Providence-based biotechnology company selected from more than 100 global research initiatives aimed at impacting drug development
Tregitope Innovation Award Press Release (PDF)
May 18, 2010 (Providence, RI; San Francisco, CA)—At the American Association of Pharmaceutical Scientists’ (AAPS) 2010 National Biotechnology Conference (NBC) in San Francisco, AAPS President Danny D. Shen, Ph.D., presented an award for “Innovation in Biotechnology” to Providence-based EpiVax; commemorating their contributions to the pharmaceutical sciences. EpiVax Tregitope research was selected from more than 100 submissions globally. The award recognizes innovation demonstrating how basic science can impact drug development and represents a creative new idea expected to have a significant impact in the biotechnology field.
“The award winners announced are a testament to the advances AAPS members and their colleagues have made in the field of biotechnology,” said AAPS Associate Executive Director Peter Inchauteguiz. “Recognizing these scientists for their hard work and achievements in biotherapeutics is a great way for us to kick-off this national conference in San Francisco.”
EpiVax award-winning research entitled, “Preclinical Design of Less Immunogenic Biologics: Tregitopes and Tolerance,” was done with the support of researchers from the following institutions: Brigham and Women’s Hospital; Children’s Hospital Boston; Children’s Hospital of Philadelphia: Institute for Immunology and Informatics, University of Rhode Island: and University of Maryland.
When asked about the significance of the Tregitope discovery, Harald Kropshofer, Ph.D, Global Coordinator Immunosafety, F. Hoffmann-La Roche AG has said “I believe that Tregitope technology represents a shift in thinking that may allow the biotherapeutics industry to address a range of unanswered questions related to side-effects based on immunogenicity; it truly has the potential to provide important advances in minimizing unwanted immune responses through tolerance induction.”
The discovery of natural regulatory T cell epitopes (Tregitopes) in the sequence of therapeutics mAbs represents a paradigm shift for protein therapeutics, allergy, autoimmunity and transplantation. Tregitopes are currently being evaluated in vitro and in vivo in five collaborating academic laboratories. For example, investigations in Transplantation (MLR, cardiac transplant model) are being carried out by Mo Sayegh and Nader Najafian (Transplantation Research Center, Brigham and Woman’s Hospital and Children’s Hospital Boston, Harvard Medical School), in protein therapeutics (FVIII) and autoimmunity (Type 1 Diabetes) by David Scott (Department of Surgery, Microbiology, University of Maryland School of Medicine), in Multiple Sclerosis by Samia Khoury (Center for Neurologic Diseases, Brigham and Women’s Hospital, Harvard Medical School), have demonstrated the Tregitopes activate natural Tregs and induce adoptive tolerance to a range of target antigens. Current studies are focusing on dose, delivery and route of administration to maximize the Tregitope effect. Safety is expected to be excellent, as Tregitopes are believed to be the active component of a widely used immunotherapy, IVIG.
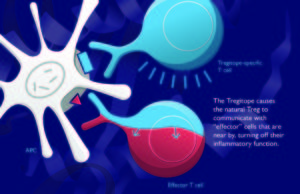
About Tregitope
Tregitopes are a set of peptides that specifically activate CD4+CD25+FoxP3+ natural regulatory T cells (nTregs). Tregitopes are promiscuous MHC Class II T cell epitopes located in the Fc and framework regions of Fab from IgG. In vitro, co-incubation of antigens with Tregitopes in vitro leads to a suppression of effector cytokine and chemokine secretion, reduced proliferation of effector T cells, and expansion of antigen-specific adaptive Tregs (aTregs). In vivo, co-administration of Tregitopes with a wide range of proteins (such as FVIII, thyroid stimulating hormone receptor, ovalbumin, and autoantigens) leads to suppression of T cell and antibody responses to test antigens.
About EpiVax
EpiVax, Inc. is a privately held company based in Providence, Rhode Island. EpiVax uses cutting edge bioinformatics tools (more specifically, immunoinformatics tools such as the EpiMatrix algorithm) to improve the efficacy and safety of vaccines and biologic products (proteins such as Humira, Factor VIII, and Botox). EpiVax also has extensive internal programs focused on developing new methods for modulating the human immune response using its Tregitope Technology platform. For more information about EpiVax, please visit www.epivax.com. For more information on the Tregitope technology and EpiVax’s other offerings please contact Jason Del Pozzo at jdelpozzo@epivax.com.