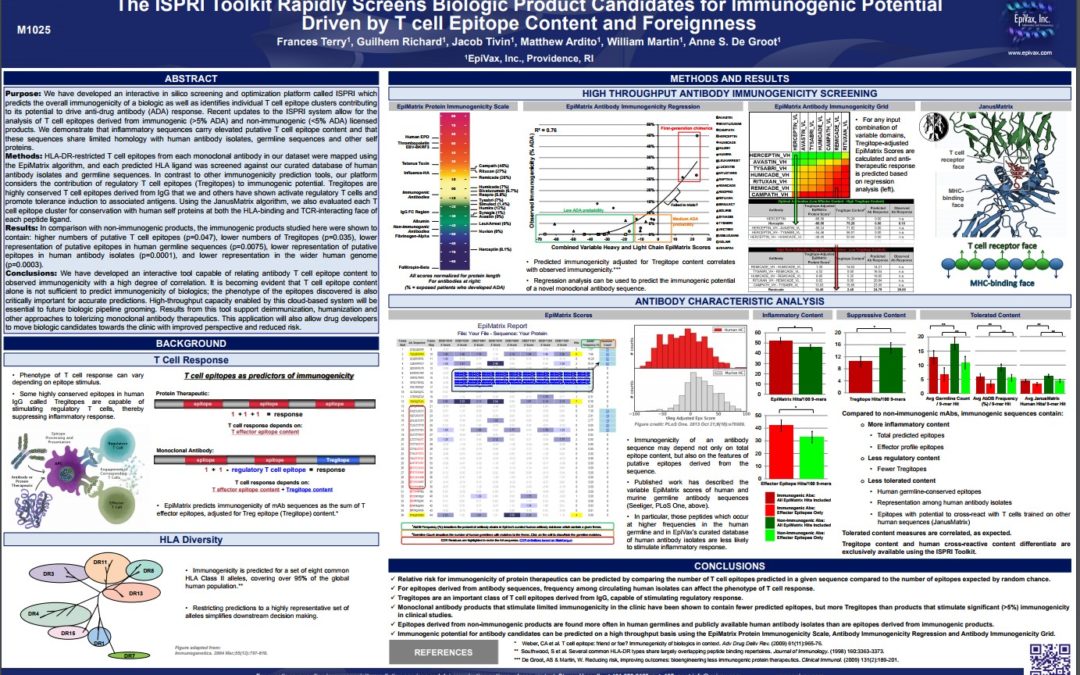

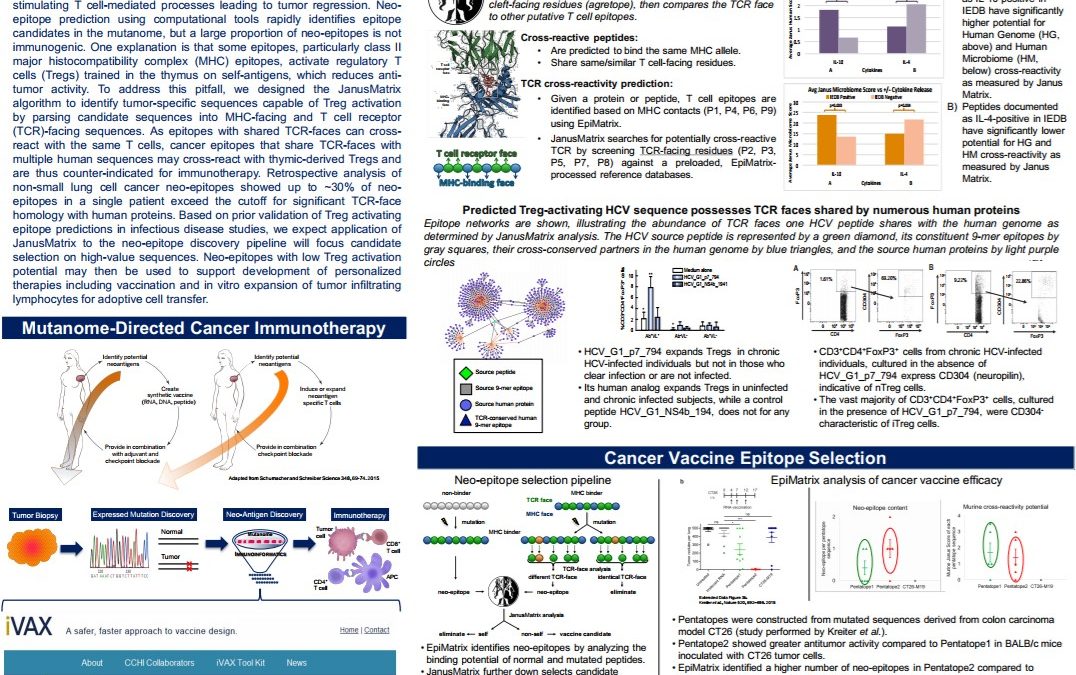
Improved Personalized Vaccine and Adoptive Cell Transfer Immunotherapy Design by Immunoinformatic Analysis of Cancer Neo-epitopes
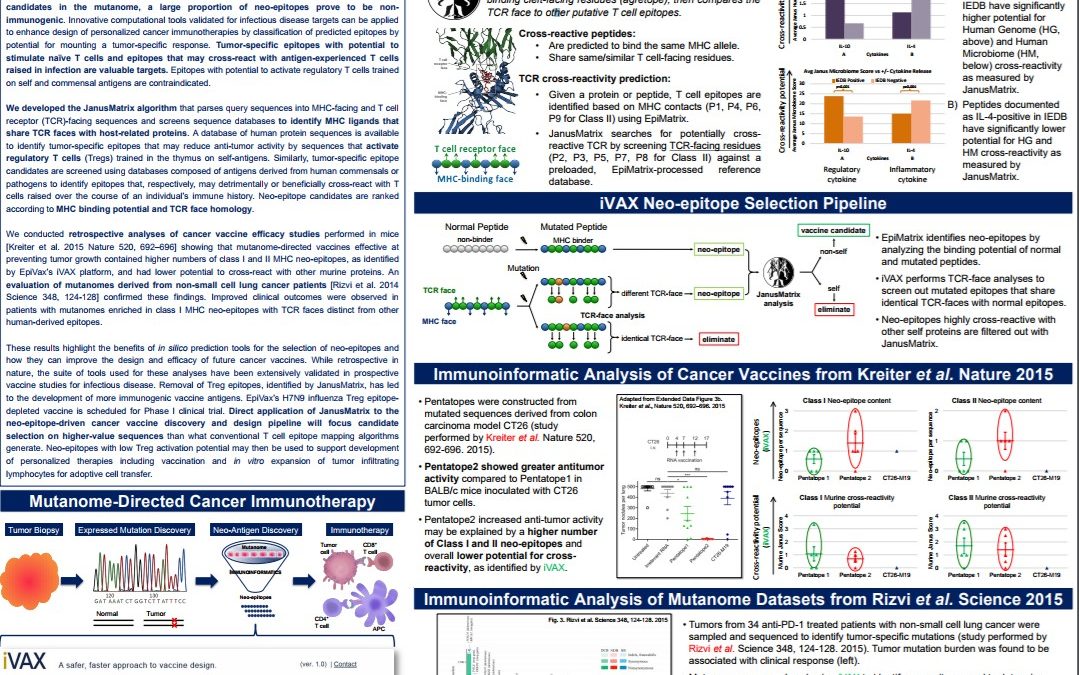
High-Value T Cell Epitope Selection for Mutanome-Directed Cancer Immunotherapy Using an Innovative Cancer Neo-Epitope Classification System
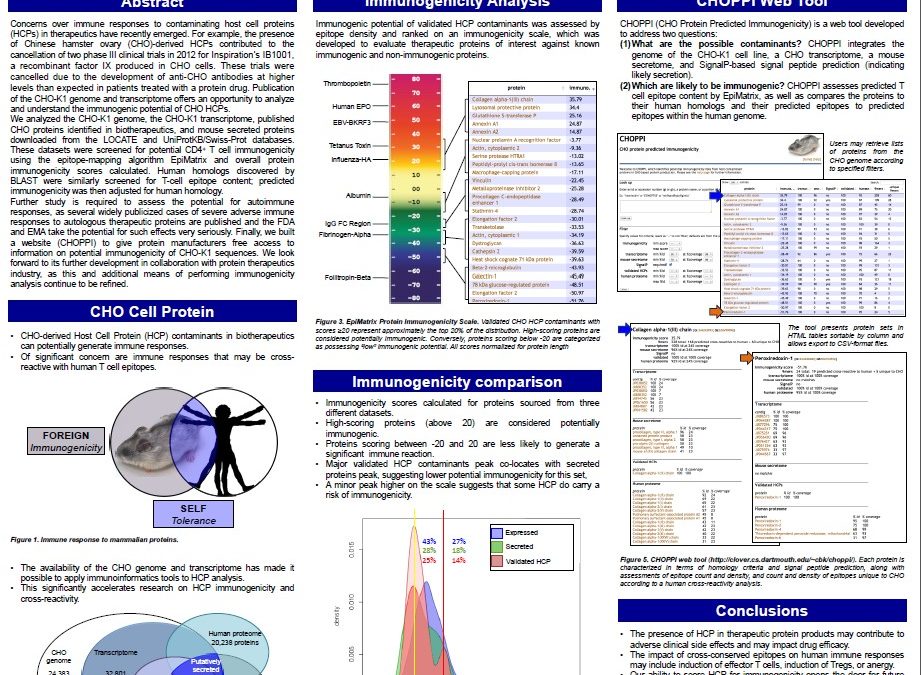
Immunogenicity Analysis of Chinese Hamster Ovary (CHO) Host Cell Protein Contaminants in Therapeutic Protein Formulations