The Curious Case of HCP-driven Immunogenicity
The FDA briefly “paused” the Johnson & Johnson adenovirus-based COVID-19 vaccine in April, and the AstraZeneca vaccine (also adenovirus-based) was in the news as early as March. Both of these pauses were to investigate rare but serious cases of blood clots (thrombotic thrombocytopenia) that had been associated with the vaccines. We have been following the emerging research related to this topic closely, ever since.
First of all, it’s important to note that vaccine-induced thrombotic thrombocytopenia (VTT) does not seem to be associated with antibodies generated against SARS-COV-2 spike proteins found in all approved COVID-19 vaccines. Instead, some scientists are honing in on Host Cell Proteins (HCPs) as a potential driver of the observed negative side effects.
And indeed, the story is getting interesting …
As reported in a recent pre-print the (AstraZeneca) ChAOx1 nCov-19 vaccine was found to contain a number of HCPs related to platelets that might drive anti-platelet immune responses. A pre-print. by a separate group of researchers also postulated that VTT might be related to host cell protein components. Typically, HEK-293 and Per.C6 lines are used to produce the live virus containing the vaccine antigen. Both the Janssen and AstraZeneca COVID-19 vaccines are produced using mammalian cell lines.
Why the focus on Host Cell Proteins (HCPs)? HCPs may be difficult to purify away from biologic products (and vaccines) and for that reason they are sometimes called ‘hitchhiker proteins“. A wide array of cell lines are used to quickly manufacture protein products and viral-vectored vaccines.
We’ve been curious about HCPs for a long time. Immune response to HCPs might be driven by T cell epitopes that are somewhat different (e.g. more foreign) than the human homologs. Immune response to these epitopes msy be more likely to occur when they are co-administered with a vaccine containing “danger signals” such as viral RNA. The foreign epitopes could then initiate epitope-spreading immune responses to human proteins (e.g. platelet proteins).
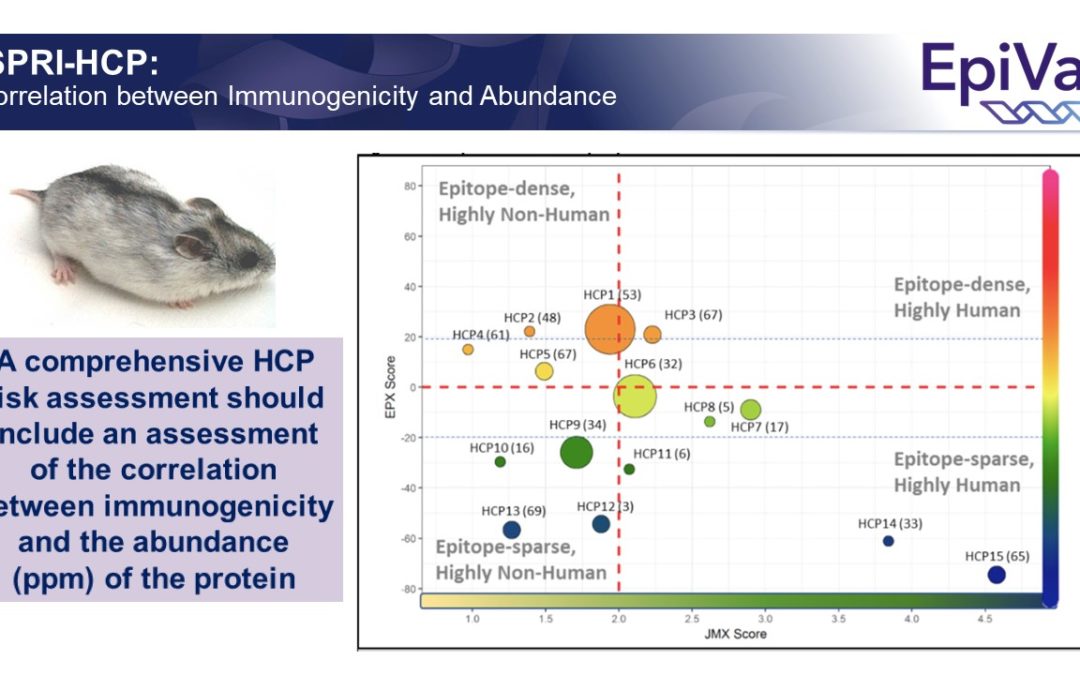
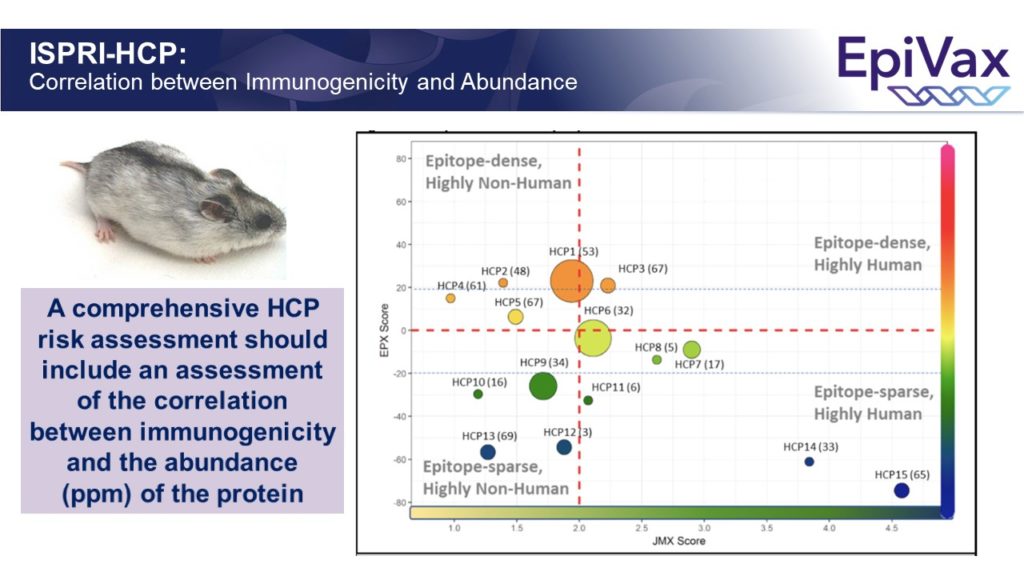
Improved identification of potentially immunogenic HCPs (and removal of those HCPs) might reduce off-target immune responses to vaccines (and biologics) developed in mammalian cell lines in the future.
And, yes, we developed an app for that! We’re happy to report that EpiVax’s ISPRI-HCP toolkit is designed to analyze HCP (see sample report below). It currently provides evaluation of CHO HCP, but can be adapted to any proprietary cell line of interest. And of course, we use…. JanusMatrix to perform the ISPRI-HCP evaluation. We’re happy to share more details on the process, so feel free to reach out!
EpiVax 23rd Birthday World Tour!
Did you know EpiVax turned 23 in May? We marked the occasion with our first in-person gathering back at the office😊. We also welcomed 6 new team members that we are finally getting to meet in person! (Here’s Happy Jack with the crew!)

And with the roll-out of vaccines getting up to speed, we will be kicking off the “Fearless Science World Tour” trip this fall, bringing back our Immunogenicity Seminar Series! Amsterdam, Tokyo, Seoul, Lisbon, Barcelona, Paris and Basel, here we come! Let us know if you are ready for a visit!
Will you be in Amsterdam on October 1st? Join us at the The Hilton Amsterdam. For the 6th annual Immunogenicity and Tolerance Seminar.

In Tokyo November 12th? Join us at the Westin Tokyo for the 14th annual Immunogenicity Seminar.

Wish we were coming to a city near you? Well let us know, and we can make it happen!
And we are excited for BIO International (virtual!) June 14th-18th. Attending? So is EpiVax’s Katie Porter! Look her up in the partner portal.