Press
Exploring the Link between Polio Vaccination and Hand Foot Mouth Disease
Posted on June 25, 2015 by Annie De GrootThe Centre for Health Protection (CHP) of the China Department of Health is investigating an outbreak of hand, foot and mouth disease (HFMD) at a residential child care center in Causeway Bay, Hong Kong. Affected children tested positive for enterovirus (EV71) which usually causes HFMD. According to researchers Qiben Leng and Annie De Groot, the failure of polio vaccine ...Read more$5.2 Million Department of Defense Grant Awarded to "Vaccine on Demand" Consortium
Posted on June 9, 2015 by Annie De GrootPROVIDENCE, RI – June 9, 2015 – The Defense Threat Reduction Agency (DTRA) is investing $5.2 Million for the development of a new type of vaccine against Q fever. Academic and industry organizations from around the world, led by Mark Poznansky of the Vaccine and Immunotherapy Center (VIC) and Massachusetts General Hospital and Annie De ...Read more5th Computational Vaccinology Workshop (CV5) at ISV
Posted on June 1, 2015 by Annie De GrootWe invite you to join us for this year’s iteration of the Computational Vaccinology Workshop @ ISV. Register for this event! Abstract submissions for full-length papers and posters are still being accepted – submit your abstracts now. The 5th Computational Vaccinology Workshop at International Society for Vaccines (CV5 @ ISV) is a satellite workshop to be held Saturday, October 17th ...Read moreTestimony on Faster, Safer, More Effective Vaccines by Dr. Annie De Groot to the Blue Ribbon Panel on Biodefense.
Posted on April 10, 2015 by Annie De GrootAnne De Groot, CEO of EpiVax, will testify before the Blue Ribbon Study Panel on Biodefense about encouraging innovative methods for solving existing and new biothreats. View a PDF of Annie’s testimony.Read moreURI Accepting Fellows for NTD Vaccine Design Toolkit and Training Workshop Set for May 2015
Posted on March 29, 2015 by Annie De GrootPROVIDENCE, R.I. – March 26, 2015 – The University of Rhode Island’s Institute for Immunology and Informatics is currently accepting fellows to participate in a workshop and training course targeting neglected tropical diseases (NTD) and emerging infectious diseases (EID). The workshop will focus on training NTD/EID researchers interested in using new vaccine design tools developed by Principal ...Read moreWestin Immunogenicity Seminar Seoul – April 2015
Posted on March 28, 2015 by Annie De GrootUPDATE: April 28th, 2015. Here are the slides that EpiVax presented at the seminar: Vaccine Design Using in Silico Tools (6.4MB) Immunogenicity Screening and Epitope Engineering (6.3MB) We had a great time in Seoul and we hope you did also! Thank you for your participation and engagement. If you have any questions please email info@epivax.com for a prompt reply. We hope to ...Read moreWhere BLAST fails . . . JanusMatrix succeeds!
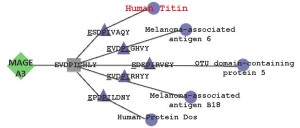
Posted on March 26, 2015 by Annie De GrootWhen two patients died* due to cardiac complications following treatment with an immunotherapy (T cells armed with a chimeric antigen receptor for a Mage A3 peptide), researchers checked the target peptide again. Using BLAST, there was no obvious cause: no cross-reactivity with any human protein was detected. But where BLAST failed, a new algorithm was able to clarify the relationship ...Read morePersonalized Medicine for Biologics?
Posted on March 5, 2015 by Annie De GrootWhich one is not like the other? Why is one patient more likely to develop a side effect like anti-drug antibodies (ADA) than another? While there are many contributors to immunogenicity, most developers agree that T cell epitopes are key, and because they are, our genetic make up (HLA) determines who will, and who won’t ...Read moreSuccessful feasibility studies drive new Novozymes-EpiVax agreement
Posted on December 22, 2014 by Annie De GrootEpiVax Inc. enters license agreement with Novozymes Biopharma to advance pioneering treatment of autoimmune diseases Novozymes’ Veltis® albumin-based half-life extension platform will help move Tregitope treatment for autoimmune diseases closer to clinical development. Providence, RI. Monday December 22, 2014 EpiVax and Novozymes Biopharma today announced that they have entered a license agreement for the development of novel ...Read moreAntigen Specific Tolerance Induction at Immunogenicity Summit Nov 19 2014
Posted on November 18, 2014 by Annie De GrootWhy is antigen-specificity key for biologics? Annie De Groot, MD, CEO and CSO of EpiVax will be speaking at the CHI Immunogenicity summit on this topic, November 19th at 3 PM. It’s well known that tolerance can be induced by powerful transplant drugs but combining those powerful agents with biologics would not be generally acceptable (certainly not ...Read moreThe Story-Telling-Cloth – Or how we use Textiles as Social Media to improve Vaccine Uptake
Posted on November 4, 2014 by Annie De GrootGAIA Vaccine Foundation’s Story-Telling Cloth receives “Grand Challenges Explorations Grant” Award For West African-Style “Social Media” Cervical Cancer Prevention Campaign Providence, Rhode Island, 04 November 2014. The Bill and Melinda Gates Foundation awarded $100,000 to GAIA Vaccine Foundation to test whether dissemination of a printed cloth that tells the story of HPV, coupled with a media campaign ...Read moreChugai Licenses ISPRI and OptiMatrix Platform from EpiVax for De-Risking Biologics
Posted on October 29, 2014 by Annie De GrootProvidence, Rhode Island. October 30, 2014 Chugai Pharmaceutical Co. signed an agreement with Rhode Island-based biotechnology company, EpiVax, Inc., to incorporate EpiVax’s ISPRI immunogenicity screening and deimmunization technology into Chugai’s drug development toolbox. Chugai is widely regarded as one of the most cutting edge biopharmaceutical companies in Japan, Researchers at Chugai will be utilizing the cloud-based in ...Read moreAnd . . . Why **Don't** we have an Ebola Vaccine Yet?
Posted on October 21, 2014 by Annie De GrootEbola: It is better to Prevent than to Cure These days, every conversation starts with this question: Why don’t we have a vaccine against Ebola? Francis Collins, Director of the National Institutes of Health, gave the right answer in a recent interview: “Frankly, if ...Read moreSenator Whitehouse, Homeland Security Headline Vaccine Renaissance Conference
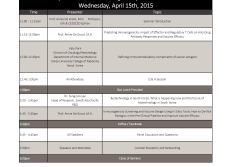
Posted on October 14, 2014 by Annie De GrootSenator Whitehouse (RI) addressed more than 100 experts in the field of vaccine development today at the 8th Vaccine Renaissance, an annual event organized by the University of Rhode Island’s Institute for Immunology and Informatics and supported by EpiVax. He stated that it was tragic that the Sequester achieved what it set out to do – to be a ...Read more8th Vaccine Renaissance October 13-16 2014
Posted on October 8, 2014 by Annie De GrootVaccine experts to gather in Providence as fight against deadly diseases carries on Providence, R.I. – October 8, 2014 – As many African countries as well as the United States continue to grapple with the outbreak of Ebola and other deadly diseases, the University of Rhode Island is getting ready to play host to some of ...Read more
Join our newsletter
We strive to provide our clients, collaborators and everyday immunology enthusiasts with valuable content. Written by our CEO/CSO Dr. Anne Dr Groot, you can expect up to date vaccine development efforts, upcoming events and so much more from our offices here in Providence, RI.