Developing biologics? EpiVax’s High Throughput Immunogenicity Assessment provides a comprehensive assessment of potential clinical immunogenicity for large sets of biologic candidates.
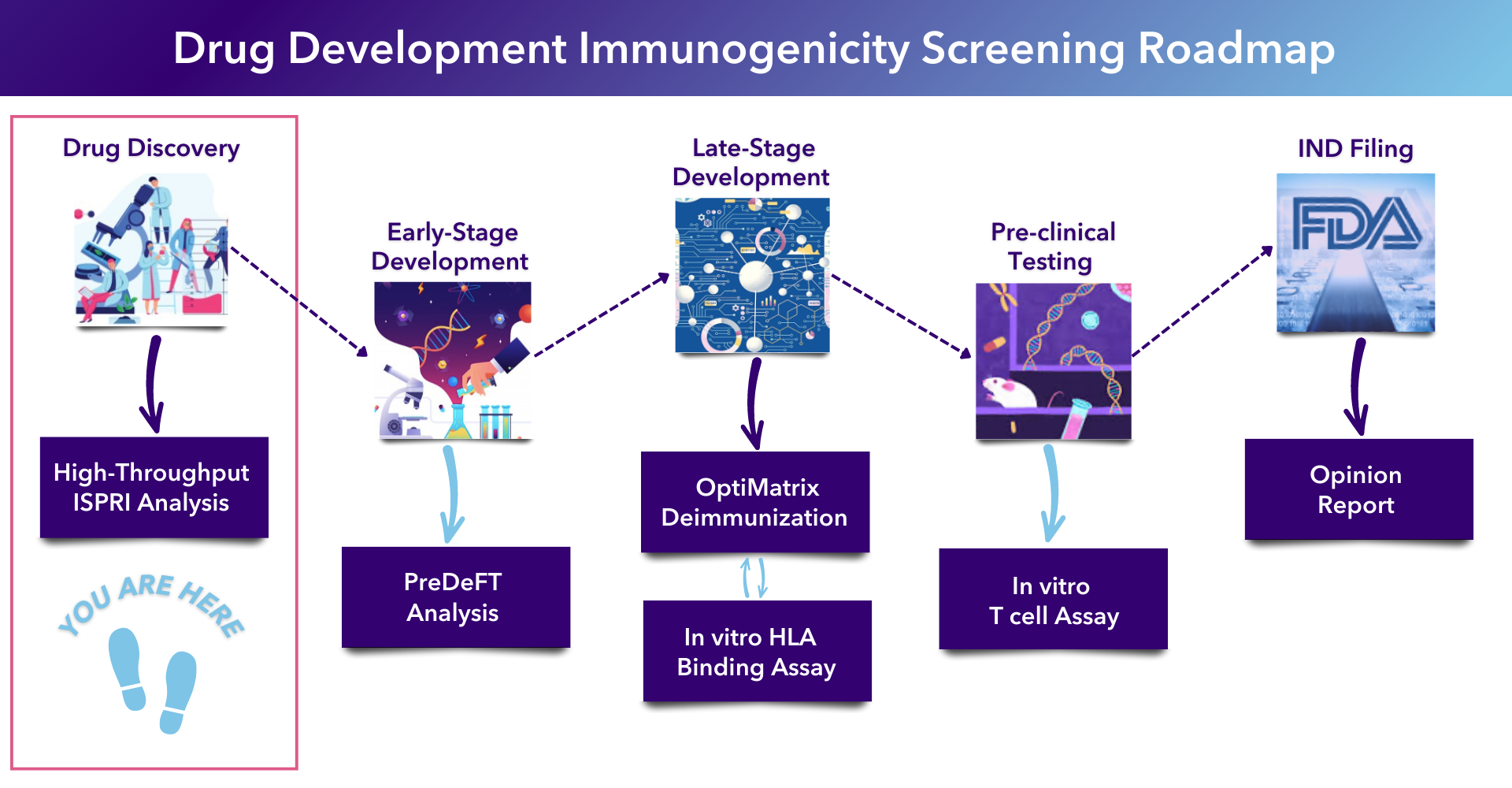
This approach allows preclinical researchers to compare the immunogenic potential of a large set of proteins or antibody-derived sequences and acts as a mechanism for identifying the best lead candidate(s) to bring forward into development.
The analysis is performed by the immunoinformatics experts at EpiVax using the in silico options within the Interactive Screening and Protein Reengineering Interface (ISPRI) Toolkit. The analysis is summarized in a tabulated report, including visual aids detailing the immunogenicity of the proteins of interest.
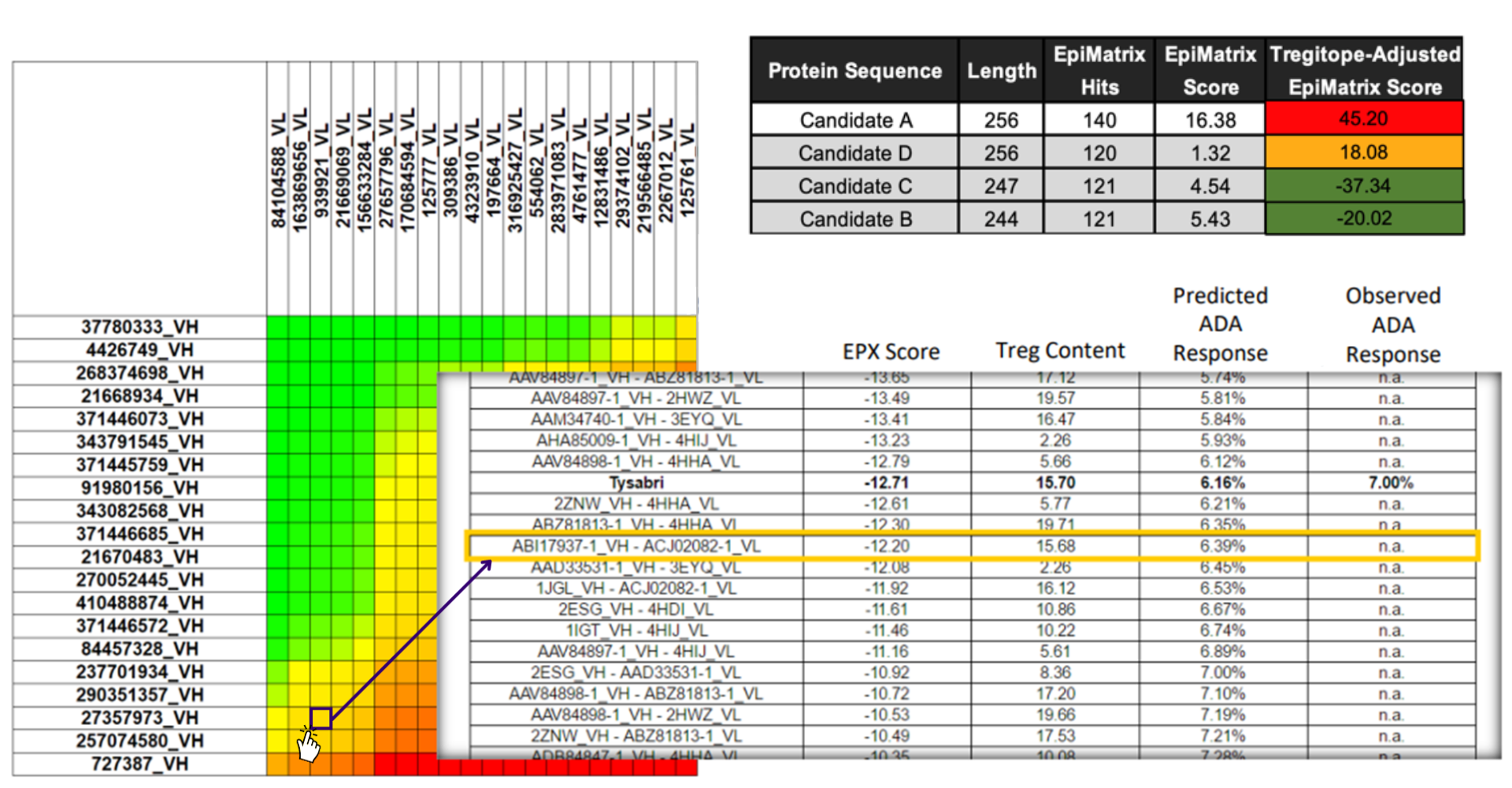
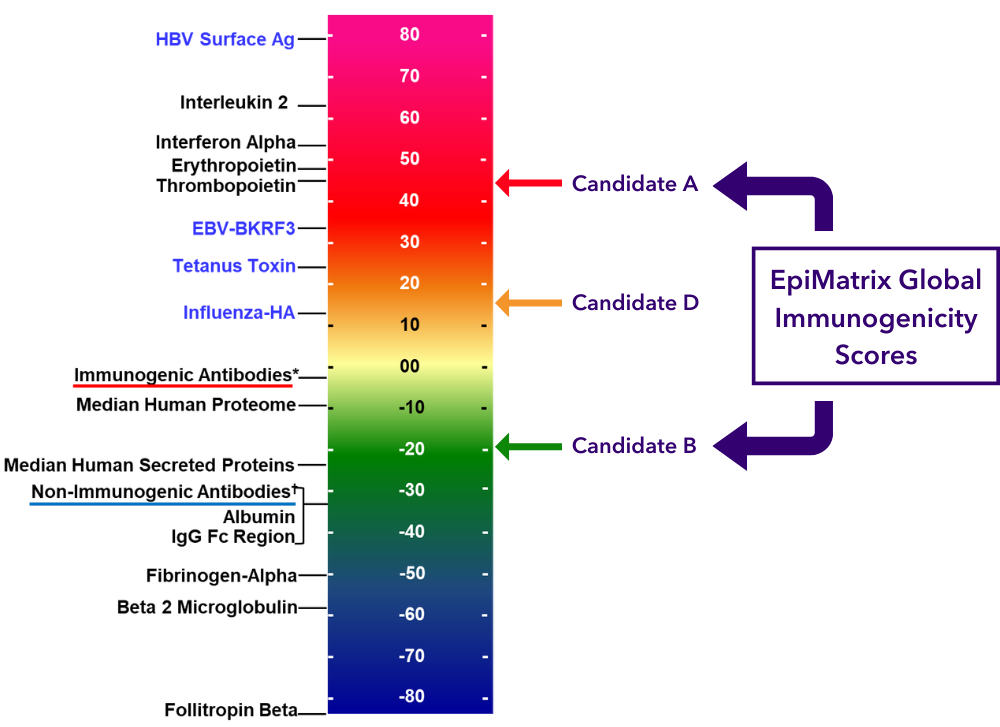
As shown in the Antibody Immunogenicity Scale below, ADA is estimated and ranked alongside a panel of licensed antibodies.
What are the Benefits of High-Throughput Immunogenicity Analysis During Preclinical Activities?
- Reduce your laboratory work (on average, more than 20-fold) and focus development on critical protein regions.
- Assess immunogenic potential for nine different human HLA DR supertypes, which combined cover more than 95% of the human genetic variability across the globe.
- Save time, money, and effort by providing your team with actionable data on protein immunogenicity.
Here are some recent papers from the industry referencing the benefits of ISPRI as an immunogenic risk assessment strategy:
- Implementing a Clinical Immunogenicity Strategy using Preclinical Risk Assessment Outputs – Journal of Pharmaceutical Sciences (jpharmsci.org)
- Frontiers | T-Cell Dependent Immunogenicity of Protein Therapeutics Pre-clinical Assessment and Mitigation–Updated Consensus and Review 2020 (frontiersin.org)
For more information on High-Throughput Antibody Analysis or other EpiVax tools, contact our business development team:
Sarah Moniz – smoniz@epivax.com
Riley Nolan – rnolan@epivax.com
Sameer Laul – slaul@epivax.com