Gene Therapies – time to think about Immunogenicity
I just returned from the Gene Therapies for Rare Disorders meeting in Boston… and wow, what an exciting time for these therapies! Here’s a good overview of AAV therapies currently approved and in clinical trials for rare disease.
Of course it begs the question… what is the immunogenic risk of these new products? Data is just starting to emerge, and it appears there can be risks that should be investigated.
So of course I had to run a published sequence through EpiVax’s ISPRI toolkit!
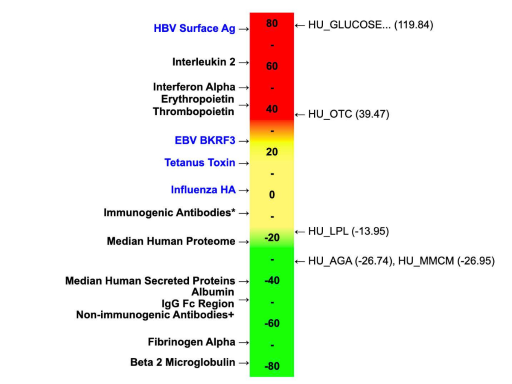
Glucose-6-phosphatase deficiency is a rare inherited glycogen storage disease. AAV therapies are currently in development for G6P deficiency, to insert a functional version of catalytic subunit G6P-alpha into patients. As you can see below, Human Glucose-6-phosphatase scores quite high on our protein immunogenicity scale.
Of course the scale doesn’t tell the whole story, and we have additional EpiVax tools (homology analysis with JanusMatrix for example) that could make this less of a risk than the high EpiMatrix score might indicate. But to my eye, this is something that needs to be further characterized!
Oh and EpiVax had a poster at the conference… “Immune Tolerance-Adjusted Personalized Immunogenicity Prediction for Rare Diseases”, which focuses on our Personalized Immunogenicity Risk Assessment (PIMA) for Pompe Disease.
Working on gene therapy and interested in characterizing your immunogenicity risk? Get in touch!
Tregitope Summit

I am pleased to share that EpiVax will be hosting the 1st Annual Tregitope Summit on May 16th in Boston!
I’m organizing this meeting with Dr. Amy Rosenberg (Formerly FDA, now EpiVax), and Dr. David Scott (Uniformed Services University of the Health Sciences) who will each chair a session.
Interest in therapeutics specifically for inducing and activating Tregs by various approaches including administration of Tregitopes, is growing, and we see the need for an annual meeting to provide a space for academic and industry researchers to share ideas, create new collaborations and continue to expand this growing therapeutic area.
Interested in attending? Register here! https://bit.ly/EpiVaxTregitopeSummit
For convenience, we planned this event for the day before Treg-directed Therapies (Also in Boston!). Amy and I will both be presenting there as well, on the below topics.


Women in Biotechnology Summit, NYC
If you’re connected with me on LinkedIn, you’ve probably seen some of the recent initiatives at EpiVax to help the next generation of #WomenInScience. Although we’ve collectively come a long way on many gender inequity issues, there is LONG way to go. Did you know that although women now make up roughly 50% of life sciences undergrads, the number of women in C-level roles at biotechnology companies is only 6%!
This month Katie Porter and I hosted the inaugural Women in Biotechnology Summit at the lovely Edition Hotel in NYC.
This gathering of women biotechnology leaders sparked a lively discussion on how we can support early career women scientists. All participants left with a ten point plan to implement for their companies and their own career path!
The conversations from this meeting will continue on our “Recipes for Success” podcast, so stay tuned for several new guests this spring!
Subscribe on Apple Podcasts: https://podcasts.apple.com/us/podcast/recipes-for-success/id1602397850
March is PANDA Month!
EpiVax has a unique immunogenic risk assessment for generic peptide developers submitting an Abbreviated New Drug Application (ANDA) with the FDA. We’ve done hundreds of these Peptide Abbreviated New Drug Application (PANDA) screenings since 2017 when we first developed the program. And of course, we absolutely HAD to feature a Giant Panda for our logo😊

To celebrate 5 years of PANDA screenings, and to do our part for Wild Panda conservation, EpiVax is supporting the World Animal Protection League this March, through our VaxGivesBack initiative.
Did you miss this month’s newsletter? Sign-up here



